
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

December 29, 2023
What a year! Perspectives has explored a myriad of topics this year within clinical development — from adaptive trial...
Read article

December 22, 2023
Top Therapeutics Development Topics of 2023
Perspectives covers a wide range of topics within therapeutics development from advice on regulatory submission to...
Read article

December 23, 2021
Year-End Roundup: Your Favorite Blog Posts of 2021
Cytel blogs bring you debate and discussion of the newest trends in statistics and quantitative strategy. In 2021, our...
Read article

June 9, 2021
Selecting a Clinical Trial Design: How Broadly Should You Explore?
When selecting clinical trial designs, how many design options should a sponsor explore? Would a sponsor feel more...
Read article

June 2, 2021
A Data-Infused Approach to De-Risking Clinical Trials
For many decades the Pareto Frontier has been employed by actors in the private sector to evaluate and understand the...
Read article

December 21, 2020
Year-End Roundup: Your Favorite Blog Posts of 2020
2020 has been an unusually difficult year as the global pandemic impacted all of our lives. This year, the Cytel blog...
Read article

December 15, 2020
Satisficing, Optimizing and Globally Optimizing Trial Designs
When designing clinical trials, biostatisticians and clinical development teams are often faced with a conundrum. Given...
Read article
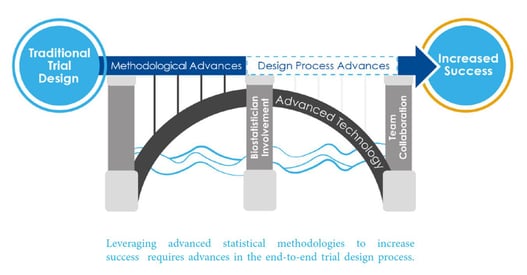
December 9, 2020
7 Key Features of Strategic Clinical Trial Design
As a part of Cytel’s Advanced Design Framework, a new Framework for the statistical design of clinical trials, Cytel...
Read article
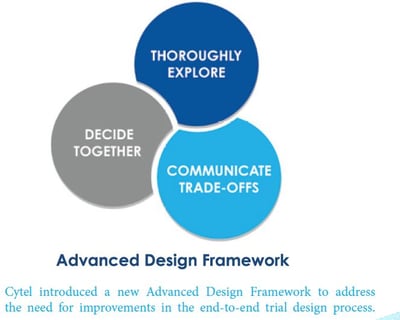
December 3, 2020
New Whitepaper: Reimagining Clinical-Trials
Increasing Clinical Development Productivity Using Statistics and Cloud-Computing The need for Re-imagining Clinical...
Read article

November 18, 2020
We can design over 100,000 clinical trials in less than an hour
The current state of the clinical trials industry faces a challenge that was only hypothetical three or four years ago....
Read article

November 11, 2020
Interview with Yannis Jemiai: Advanced Design Framework
The widespread use of cloud-computing has altered the clinical trial design process. Whereas three or four years ago,...
Read article
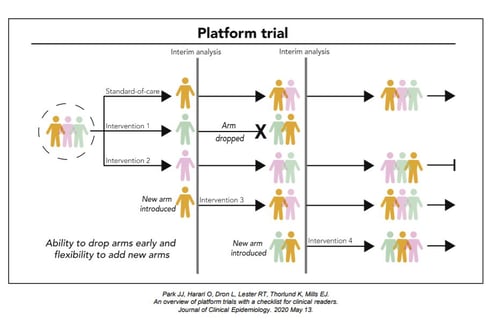
November 10, 2020
Key Design Considerations for Platform Trials
Platform trials are a new type of clinical trials where multiple interventions can be evaluated simultaneously against...
Read article

November 4, 2020
Cytel Introduces Advanced Design Framework: Part 3 - Communication Techniques to Ensure Alignment on Data-Driven Clinical Trial Designs
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought leaders that draws on...
Read article

October 29, 2020
Advanced Design Framework: Part 2 - A Quantitative Evaluation Approach
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought leaders that draws on...
Read article

October 21, 2020
Advanced Design Framework: Part 1 - Exploration of Design Space
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought-leaders after a decade...
Read article

October 15, 2020
An Advanced Design Framework for Clinical Development in the Era of Cloud-Computing
For over a decade, advanced trial design techniques have promised efficient trials with accelerated timelines,...
Read article

October 9, 2020
Cyrus Mehta on Increasing the Power of Platform Trials
Even before the era of COVID-19, significant attention was channeled to the overwhelming potential of adaptive MAMS...
Read article
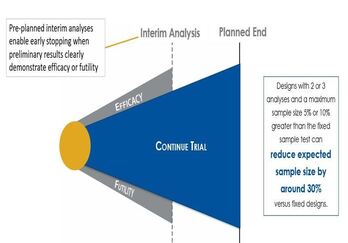
October 1, 2020
Improve Trial Design with Sequential Design and Sample Size
Methods involving Group Sequential Designs are one of the earliest deviations from a traditional two-arm clinical trial...
Read article

September 28, 2020
Advantages of platform designs for investigating COVID-19 therapies
Cytel has recently designed and implemented the TOGETHER Trials, funded by the Bill & Melinda Gates Foundation to...
Read article

September 21, 2020
Novel Adaptive Platform Trial for COVID-19 Therapies
Cytel has designed and implemented a novel adaptive platform trial for early stage COVID-19. The severity of the...
Read article

July 27, 2020
Introduction to Population Enrichment by Dr. Thomas Burnett
Cytel is conducting a webinar series on complex innovative trial designs. Dr. Thomas Burnett, Senior Research Associate...
Read article
June 11, 2020
Adaptive Bayesian Methods: The Secret Weapon in COVID-19 Vaccine Development
A recent Cytel panel led by Vice President of Strategic Consulting Natalia Muhlemann evaluated the role that Bayesian...
Read article
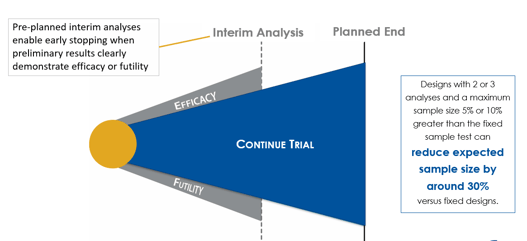
June 10, 2020
Group Sequential Designs and Sample Size Re-estimation
Cytel is conducting a webinar series that introduces biostatisticians to some of the more commonly used complex...
Read article

May 27, 2020
Group Sequential Designs and Sample Size Re-estimation
In this blog, we speak with Christopher Jennison, Professor of Statistics at the University of Bath, UK. Professor...
Read article

May 7, 2020
COVID-19: Trials, Designs and Tools for Promising Results - A Virtual Panel Discussion
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread...
Read article
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 22, 2020
What could you accomplish with a fresh approach to your clinical data strategy?
In the quest for clinical success, we all strive for evidence packages of the highest quality. If the clinical data is...
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article

January 8, 2020
How to optimize your data strategy to drive success in clinical development
In clinical development, data is the vital ‘foundation’ that supports your programs. To successfully bring a promising...
Read article

December 18, 2019
Year-End Roundup: Your Favorite Blog Posts of 2019
With only two weeks left for this fabulous year to end, we would like to thank all our blog subscribers and new readers...
Read article

August 23, 2019
Advancing Medicines Development with External Controls
In place of collecting data from patients recruited for a trial who have been assigned to the control or...
Read article

August 14, 2019
Keeping Clinical Trials on Track: A Statistician's Perspective
This article was originally published as part of a series by pharmaphorum in association with Cytel and is reproduced...
Read article

July 22, 2019
Adaptive Design and Health Economic Analysis: Interview with Laura Flight
Health economics and adaptive design methods share common ground in that they both aim to support more efficient and...
Read article

June 14, 2019
Flipping the paradigm-how should biotechs harness adaptive trials?
This article was originally published as part of a series by pharmaphorum in association with Cytel and is reproduced...
Read article


February 8, 2019
Publication Reveals New Promise for Promising Zone Designs
A 2018 publication in the Biometrical Journal by Cytel’s Cyrus Mehta, Lingyun Liu and Sam Hsiao, ‘Optimal Promising...
Read article

November 5, 2018
New publication addresses critical issues in ultra-orphan indications
Cytel biostatisticians Cyrus Mehta and Lingyun Liu, together with Charles Theuer, CEO of TRACON Pharmaceuticals have...
Read article

September 7, 2018
Opportunities of FDA’s Innovative Trial Design Pilot Meeting Program
On August 29th 2018, the FDA announced (1) that it would be establishing a Complex Innovative Trial Design (CID) Pilot...
Read article

March 16, 2018
Career Perspectives: Interview with Benjamin Esterni, Principal Biostatistician
At Cytel we believe that expert statistical input has the power to shape the future of clinical development: de-risking...
Read article

February 27, 2018
Congrats to Lipopharma and CLINGLIO Consortium on Recent Grant Award
We extend our congratulations to Lipopharma and the CLINGLIO project consortium on their recent 6,15M€ grant award by...
Read article

February 15, 2018
A Gatekeeping Procedure to Test a Group Sequential Design
A recent publication in Biometrics ‘A Gatekeeping Procedure to Test a Primary and a Secondary Endpoint in a Group...
Read article

March 2, 2017
Case Study: Bayesian Decision-Making in a Phase 3 Oncology Design
We continue our case study series with this example of a Phase 3 design that uses Bayesian decision making combined...
Read article

February 9, 2017
Inside an Oncology Statistician's Toolkit
In this blog, Adam Hamm, PhD, Director Biostatistics at Cytel shares some of the most important knowledge he uses in...
Read article

January 30, 2017
Accelerating development with combined SAD/MAD approach
Single ascending dose (SAD) and multiple ascending dose (MAD) studies are typically the first in human studies. They...
Read article

January 23, 2017
How to get the regulatory green light for your adaptive design?
As a group, Cytel had over 40 successful regulatory interactions last year, many of which supported approvals for...
Read article

January 16, 2017
Adaptive Design Approaches from Cardiovascular Clinical Trialists Forum
The Global Cardiovascular Clinical Trialists Forum is a key event bringing together leading experts from across the...
Read article

December 15, 2016
Adaptive Design CONSORT Extension Project: The Inside Scoop
In April, we interviewed NIHR research fellow Munya Dimairo about the paper, ‘Adaptive designs undertaken in clinical...
Read article
December 2, 2016
Innovative Phase 3 Adaptive Enrichment Design in Oncology
At a recent Pfizer/ Cytel seminar on rare disease and oncology development, Cytel’s Lingyun Liu presented innovative...
Read article

November 21, 2016
Infographic: 10 steps to consider before choosing an adaptive design
While adaptive designs can deliver significant benefits to clinical development- including ethical benefits for...
Read article

November 9, 2016
New East Insights Video:Creating an SSR Design
Adaptive sample size re-estimation designs are an important part of the statistician's toolkit. In this first in a...
Read article
November 1, 2016
Pharmacometrics tools of the trade: 4 factors to consider
Unlike statistics which has been around in some form for hundreds of years, pharmacometrics is, by comparison, a...
Read article

October 7, 2016
Adaptive Designs: A Data Management Perspective
Adaptive designs have the potential to accelerate clinical development, and improve the probability of trial success....
Read article
October 5, 2016
Challenges in Neuroscience Clinical Trials
While some progress has been made in terms of scientific development in Neuroscience and Neuropsychiatry indications,...
Read article

September 29, 2016
Case studies:Learning from less-well understood adaptive designs
A paper "Best practices case studies for 'less well-understood' Adaptive designs", has been published by the DIA...
Read article
September 9, 2016
Case Study:Seamless Phase 2/3 Design in Rare Disease
Challenge: Our client, an emerging biotechnology company, was preparing for the next stage of development for their...
Read article

September 7, 2016
Overcoming challenges of 'Less Well Understood' Adaptive Designs
In the 2010 draft FDA ‘Guidance for Industry on Adaptive Design Clinical Trials for Drugs and Biologics', the agency...
Read article
August 31, 2016
An Introduction to BLRM
Traditional rule-based approaches to dose escalation such as 3+3 are widely used in early clinical development. They...
Read article

August 26, 2016
Sample Size Re-Estimation in Bioequivalence Trials with Small Samples
At the recent JSM in Chicago, Cytel’s Sam Hsaio and Lingyun Liu alongside Genentech's Romeo Maciuca, presented a...
Read article

August 23, 2016
How does the T-Statistic stack up for finding MTD?
At the recent JSM meeting in Chicago, Cytel's Jim Bolognese presented the results of work he has conducted evaluating...
Read article

August 17, 2016
Adaptive Designs: In Conversation with the NEJM
Following the recent publication of their review article Adaptive Designs for Clinical Trials in the New England...
Read article
August 9, 2016
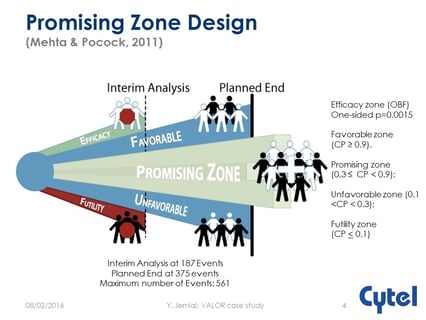
Operational and regulatory considerations in a promising zone trial
At the recent JSM Meeting, Cytel’s Yannis Jemiai presented the case study of the VALOR trial which used a promising...
Read article

June 16, 2016
Determining the future course of your trial
Predicting the course of a clinical trial is something which people will always want to do-whether for statistical...
Read article

May 31, 2016
EAST 6.4 Release: Interview with Yannis Jemiai
Last week, we were delighted to announce the release of East 6.4 bringing further cutting –edge approaches to the East...
Read article

April 28, 2016
Adaptive Designs in Practice
Adaptive Designs in Practice: Interview with NIHR Research Fellow Munya Dimairo NIHR and University of Sheffield...
Read article


