
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

February 22, 2022
When determining the best possible statistical design for a particular trial, large pharmaceuticals and small biotechs...
Read article

December 20, 2021
Approaches in Adaptive Group Sequential Clinical Trials
The promising zone design is an adaptive design which allows for sample size re-estimation based on the results of an...
Read article

December 8, 2021
Conditional Powers Vs Bayesian Predictive Power for Adaptive Sample Size Reassessment
Despite the debate in the scientific community on adaptive sample size reassessment (SSR), noteworthy developments have...
Read article
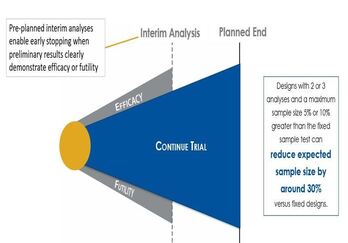
October 1, 2020
Improve Trial Design with Sequential Design and Sample Size
Methods involving Group Sequential Designs are one of the earliest deviations from a traditional two-arm clinical trial...
Read article

September 4, 2020
Cytel Co-Founder Cyrus Mehta Presents at the Heart Failure Collaboratory, a Public-Private Partnership with FDA
On Friday September 11, Cyrus Mehta, co-founder of Cytel, will be delivering a talk to the Heart Failure Collaboratory,...
Read article
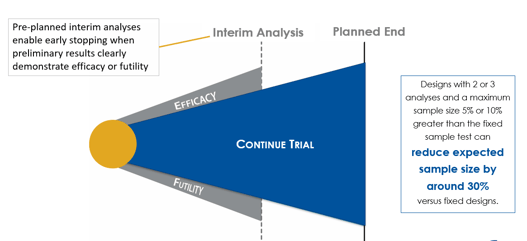
June 10, 2020
Group Sequential Designs and Sample Size Re-estimation
Cytel is conducting a webinar series that introduces biostatisticians to some of the more commonly used complex...
Read article

May 27, 2020
Group Sequential Designs and Sample Size Re-estimation
In this blog, we speak with Christopher Jennison, Professor of Statistics at the University of Bath, UK. Professor...
Read article

February 8, 2019
Publication Reveals New Promise for Promising Zone Designs
A 2018 publication in the Biometrical Journal by Cytel’s Cyrus Mehta, Lingyun Liu and Sam Hsiao, ‘Optimal Promising...
Read article

November 5, 2018
New publication addresses critical issues in ultra-orphan indications
Cytel biostatisticians Cyrus Mehta and Lingyun Liu, together with Charles Theuer, CEO of TRACON Pharmaceuticals have...
Read article

September 7, 2018
Opportunities of FDA’s Innovative Trial Design Pilot Meeting Program
On August 29th 2018, the FDA announced (1) that it would be establishing a Complex Innovative Trial Design (CID) Pilot...
Read article

April 11, 2017
FDA 22 Case Studies and Mitigating Phase 3 Risks
In a January 2017 paper (1), the FDA reviewed 22 case studies where promising Phase 2 trials did not result in...
Read article

March 10, 2017
Flexible approaches to Biosimilars Development
At the recent Biosimilars Summit in Philadelphia, Cytel's Pantelis Vlachos presented on statistical challenges and...
Read article

March 2, 2017
Case Study: Bayesian Decision-Making in a Phase 3 Oncology Design
We continue our case study series with this example of a Phase 3 design that uses Bayesian decision making combined...
Read article

November 9, 2016
New East Insights Video:Creating an SSR Design
Adaptive sample size re-estimation designs are an important part of the statistician's toolkit. In this first in a...
Read article

September 29, 2016
Case studies:Learning from less-well understood adaptive designs
A paper "Best practices case studies for 'less well-understood' Adaptive designs", has been published by the DIA...
Read article

April 28, 2016
Adaptive Designs in Practice
Adaptive Designs in Practice: Interview with NIHR Research Fellow Munya Dimairo NIHR and University of Sheffield...
Read article
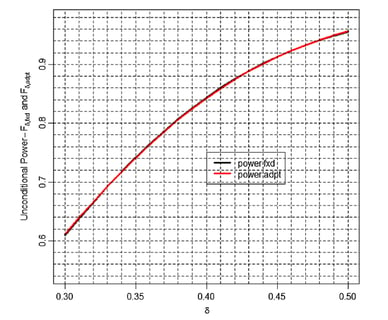
March 3, 2016
Adaptive SSR: Debunking the inefficiency myth
'The aim of a discussion should not be victory but progress.' This principle, expressed by the French essayist Joseph...
Read article


