Therapeutic Area User Guidance – The hidden Gems

CDISC standards have been around for a while with the first SDTM Standard version released in 2004. However, it was only in the last decade that it became “The Standard”, particularly when Health Authorities (HA), such as the US FDA and Japanese PMDA, made it a requirement for data submissions to support most of the regulatory requests for market approval. Additionally, most of the Pharma companies made the CDISC standards a part of their operational data model and consequently, the number of studies using the CDISC standards increased across phases of development.
The benefit of receiving data in standard formats was soon recognized by HA reviewers as they now require lesser time to understand the structure of the data they receive. Integration of data provided by different sponsors, for example on the same indication, for better understanding of safety signals, has become possible with data submitted in standard CDISC format.
However, the HAs such as the US FDA, soon realized that this was not enough, for two main reasons:
- sponsors sometimes make bad or different interpretations of the standard
- lack of standards or use cases in specific disease areas or indication
A TAUG is a guide for the implementation of CDISC standards in a specific disease or indication. Each TAUG is based on biomedical concepts that are identified by subject matter experts, and it includes examples from across CDISC foundational standards. This includes the following:
- SDTM mapping of key Therapeutic Area (TA) concepts such as disease background, endpoints
- CDASH with SDTM annotations
- Additional examples of situations not covered by the current Implementation Guideline (Ig)
- New Controlled Terminology and New Domains / New Variables might be proposed
- ADaM dataset implementation examples to support key TA endpoints
- Identification of Regulatory and Medical References
The number of available CDISC TAUGs are continuously evolving, now covering a wide range of indications, such as diabetes, oncology (Prostate, Colon, Lung and Breast), Rheumatoid Arthritis etc. As of today, since 2013, about 40 guidance have been released, with 7 new ones planned to be finalized in 2020-2021. A systematic review of the available TAUGs was presented at PharmaSUG-China 2019[5] where a review of the content of all TAUGs was made. Figure 1 shows the topics covered by each TAUG; as it can be observed from the figure, all guidance cover SDTM-related topics, while only recent TAUGs contain recommendation and use-cases for ADaM implementation (the TAUGs are presented in order of Date of First Release).
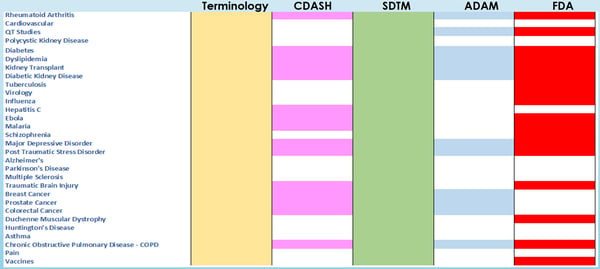
Figure 1: Standards Covered by the available TAUGs – Ordered by Date of First Released Version
Figure 2 (below) summarizes the main SDTM domains discussed in the available TAUGs. The most discussed topic is the mapping of the disease diagnosis related information in the MH domain (see also presentation from 2015 by Johannes Ulander and Niels Both[6]).

Figure 2: Key SDTM domains used in the available TAUGs
One common misunderstanding with TAUGs is that people think the recommendations and use-cases provided in the guidance apply only to the concerned TA and that is probably true when the use-cases are covering topics that are common to the TA in particular e.g. a specific disease concept or endpoint. Because of that, at least in my experience, people do not often consult or follow recommendations available in the TAUGs. However, the TAUGs contain good examples that could be applied in the same way in any other TA and these recommendations do not contradict or violate the standards but “simply” provide a “peer” interpretation of the standard. Sandra LaTorre provided some examples in her presentation at the recently held CDISC-EU 2020 Virtual Interchange[7] where she demonstrated how some SDTM mapping issues addressed in some TAUGs are valid not only for the concerned TA, but can be generalized to other TAs. For example, mapping of Diary data in FA domain and the proposed Non Standard Variables (NSVs), can be generalized and used in other clinical trials regardless of the TAs.
In several instances, the recommendations provided in individual TAUGs become either a new standard SDTM domain in the next versions of the SDTM implementation guidance or the use of specific-TA domains become of general use, as we see in the case of “RS-Disease Response and Clin Classification” that was originally developed to store Oncology Tumor Response Assessment e.g. RECIST, the domain has now a broader scope and it can be used “for the assessment of disease response to therapy, or clinical classification based on published criteria”.
The TAUGs are a great addition by CDISC to their set of standards and their main purpose is to reduce variability and space of interpretation, when applying the standards in different TA by different sponsors. This helps reduce the variability across studies of the same type. TAUGs are a great learning tool to improve your CDISC skills and clinical data knowledge regardless of whether you are working on any of the TAs supported by the available TAUGs.
References
[1] US FDA Study Data Technical Conformance Guide
[2] Japan PMDA Technical Conformance Guide on Electronic Study Data Submissions.
[3] CDER/CBER’s Top 7 CDISC Standards Issues
[4] CDISC TAUGs
[5] A. Tinazzi,” A Systematic Review of CDISC TAUGs”, PharmaSUG-China, 2019, Shanghai[6] J. Ulander and N. Both, “Therapeutic Area standards and their impact on current SDTM implementations” PhUSE-EU, 2015, Vienna
[7] S. LaTorre. “SDTM: Let’s read outside the Bible”, CDISC-EU Interchange, 2020, Virtual Conference
About Angelo Tinazzi
 Angelo Tinazzi is Senior Director, Statistical Programming, Clinical Data Standards and Clinical Data Submission at Cytel. He is a well- published and recognized expert in statistical programming with over 20 years' experience in clinical research. The application of CDISC standards in different therapeutic areas is part of his core expertise since 2003 in particular in the context of data submission to health authorities such as the FDA and PMDA.
Angelo Tinazzi is Senior Director, Statistical Programming, Clinical Data Standards and Clinical Data Submission at Cytel. He is a well- published and recognized expert in statistical programming with over 20 years' experience in clinical research. The application of CDISC standards in different therapeutic areas is part of his core expertise since 2003 in particular in the context of data submission to health authorities such as the FDA and PMDA.
Angelo is an authorized CDISC instructor and member of the CDISC ADaM Team as well as the CDISC European Committee where he also manages the Italian-speaking CDISC User Network.

