Strategies for Selecting New Indications for a Platform Trial

Thanks to Dr. Kyle Wathen for comments on this blog.
The increasing use of platform trials for the testing of a wide range of therapies raises new questions for trial design optimization and simulation. A challenge, however, is ensuring that strategies for selecting new indications for a platform are built into the risk-mitigation strategies that often go into optimizing trial design. In other words, a part of de-risking a platform trial requires a design that is robust and flexible for unknown indications that could be added in the future. In a recent Cytel webinar, Dr. Kyle Wathen, VP of Scientific Strategy and Innovation, examines this and related issues.
While a basket trial enables sponsors to study different interventions for a single population, and an umbrella trial enables sponsors to study the same intervention across different populations, a platform trial allows for both while also allowing for the possibility of adding new therapies over time. This means that when the platform is being designed, it is not clear which indications or populations will necessarily be tested in the future.
For each indication, a different Intervention Specific Appendix (ISA) is established by the clinical development team. Over the course of several years, many different ISAs might be executed (see figure below). Sharing of information between ISAs allows for more robust analysis, particularly if the ISAs are being executed concurrently. New populations could be added in a similar approach. Both the addition of new interventions and/or populations must be thought out, in detail, prior to starting the platform.
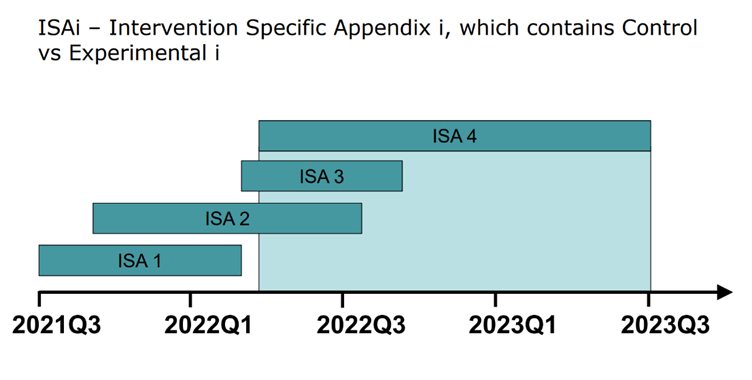
A common approach to designing a platform trial is to focus only on the first ISA. This might not optimize the trial design for indications and populations tested later. If a few different ISAs are in the pipeline, or new indications will potentially be added to the platform, it is best to meet with all the relevant stakeholders during the process of designing a trial. This will ensure that the platform is built to accommodate the various types of indications a sponsor or set of sponsors plans to test.
Using decision-rules to select new indications
A number of decision-rules need to be put in place to ensure that the selection of new indications does not adversely impact the trial. In addition to normal decision-rules like stopping for futility or efficacy or dose-selection choices, there have to be rules about when (not if) an indication ought to be added. In the case of Go/No-Go rules, it may be desirable to allow for additional information to be used that is predefined, but external to the trial. Dr. Wathen calls this “an Inconclusive Zone.”
ISAs in the inconclusive zone might indicate that in principle a new therapy may or may not benefit a population or subpopulation and external information may be utilized in order to reach a decision about the next steps in development.
Simulations can help optimize a platform trial design
Trial simulation is required to understand how a platform will perform in practice, while assessing under various scenarios. A freely available R-package called Octopus, developed by Dr. Wathen, can help sponsors design and simulate platform trials. In addition, simulation is very helpful to determine if and when a new ISA should be added to the platform. According to Dr. Wathen, optimizing a platform trial is similar to designing several trials at once, with the caveat that both benefits and challenges to one trial will have consequences for others.
To learn more about how to select indications for platform trials, and to examine other challenges confronted by trial designers, click below to access Kyle Wathen’s June 10 webinar.
About the Author of the Blog:

Dr. Esha Senchaudhuri is a research and communications specialist, committed to helping scholars and scientists translate their research findings to public and private sector executives. At Cytel Esha leads content strategy and content production across the company's five business units. She received a doctorate from the London School of Economics in philosophy, and is a former early-career policy fellow of the American Academy of Arts and Sciences. She has taught medical ethics at the Harvard School of Public Health (TH Chan School), and sits on the Steering Committee of the Society for Women in Philosophy's Eastern Division, which is responsible for awarding the Distinguished Woman in Philosophy Award.


