Cytel's blog featuring the latest industry insights.

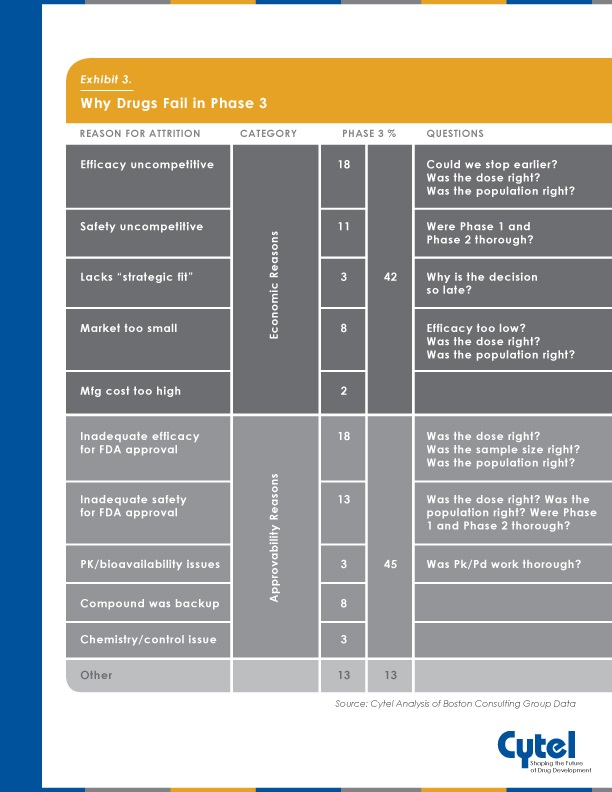
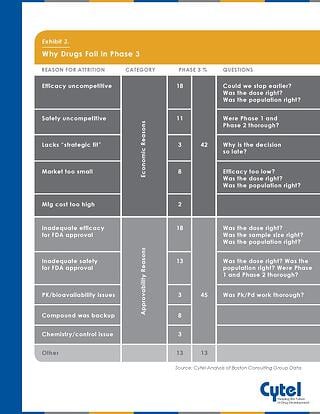
Its important to take a strategic approach to clinical development in order to minimize the potential for Phase 3 attrition. The below infographic, previously published on the blog highlights some of the approvability and economic reasons cited for Phase 3 failure , and the clinical development issues which may have had an impact.
 In a 2016 article (Bolognese et al 2016)(1) published in Therapeutic Innovation and Regulatory Science the authors including Cytel’s Jim Bolognese, Nitin Patel and Jaydeep Bhattacharyya) examine how simulations can be used to optimize a clinical trial program. Their detailed analysis explores the impact which several Phase 2 design features ( including Phase 2 sample size, decision rules to select Phase 3 dose and sample size, and number of Phase 3 trials) have on the probability of Phase 3 success and the expected net present value (eNPV) of a product.
In a 2016 article (Bolognese et al 2016)(1) published in Therapeutic Innovation and Regulatory Science the authors including Cytel’s Jim Bolognese, Nitin Patel and Jaydeep Bhattacharyya) examine how simulations can be used to optimize a clinical trial program. Their detailed analysis explores the impact which several Phase 2 design features ( including Phase 2 sample size, decision rules to select Phase 3 dose and sample size, and number of Phase 3 trials) have on the probability of Phase 3 success and the expected net present value (eNPV) of a product.
The publication, which focuses on the example of neuropathic pain, extends a framework originally discussed by Patel et al (2) . Whereas in the 2012 paper, 1 dose was selected from the Phase 2 trial results to simulate the Phase 3 trials, in the 2016 publication, the authors expand the approach with an option to take 2 doses to Phase 3, the potential to market 1 or 2 of the Phase 3 doses, and add an additional 1 or 2 Phase 3 trials based on certain criteria.
Based on the simulations, the authors concluded that taking 2 doses to Phase 3 outperforms taking 1 dose unless the weight of Phase 2 evidence strongly rules out the potential for marketing of 2 doses.
They also noted that including more doses in Phase 2 yielded generally higher eNPVs than fewer doses. This occurred especially when the true underlying efficacy and/or tolerability DR curves were at borderline levels of acceptable/optimal value.
The abstract and details of article access are available here.
To download Cytel's white paper on adaptive designs click below.
