At the recent JSM Meeting, Cytel’s Yannis Jemiai presented the case study of the VALOR trial which used a promising zone design. At the time of the study, existing therapy for relapsed or refractory AML was generally unsatisfactory with no approved drugs available for patients, and a very poor prognosis. Vosaroxin was a first-in-class anticancer quinolone derivative which had previously been studied in a single arm Phase 2 study. In this blog we'll take a look at the operational and regulatory considerations in the implementation of this trial, which were highlighted during Jemiai's talk.
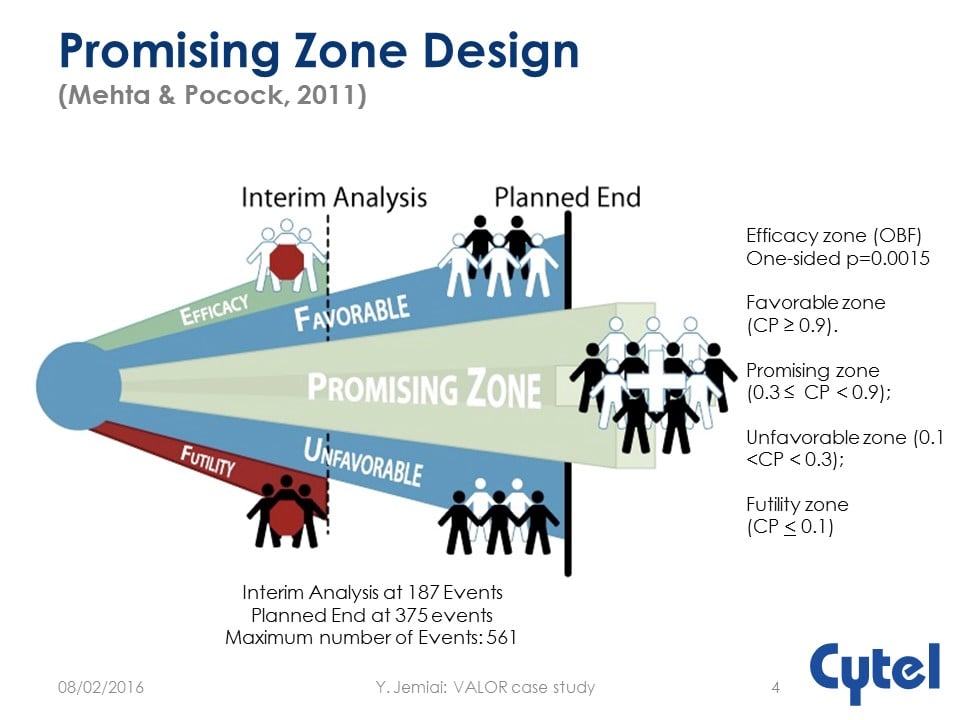
The Vosaroxin and Ara-C combination evaLuating Overall Survival in Relapsed/refractory AML phase 3 trial was a double-blind, placebo-controlled, multinational trial with Overall Survival (OS) endpoint using Two-stage Promising Zone Design.
The study was designed realistically upfront with the underlying principle of milestone based investment. It was powered to detect HR= 0.71 requiring 375 events and 450 subjects with accrual of 19 per month. There was one interim analysis taking place after 187 events with a simple adaptation rule:
- Stop early if overwhelming evidence of efficacy (LD-OBF)
- Stop early for futility if low conditional power
- Increase number of events, sample size and (if possible) rate of recruitment at the interim if results are promising
While finally failing on the primary endpoint, the totality of data suggested benefit for Vosaroxin in relapsed/refractory AML.
Regulatory Considerations
In implementing this design working closely with the regulators iscrucial. As part of the regulatory approach for such a design it is important to justify why the adaptive approach is necessary, provide a briefing document with the Statistical Analysis Plan, and comprehensively describe the statistical methodology and details for control of type-1 error. In addition simulation results under various scenarios may be provided along with a briefing document and SAP, and finally the DMC charter.
Operational Considerations
One of the important operational considerations is to ensure that the stakeholders- investigators, analysts, and investors are educated about the adaptive design. Clearly, robust and clear SOPs are critical for all trials, and no less so for an adaptive trial. These should document ' who saw what and when' ; all the data and programs used for the interim analysis and details of who has access to the adaptive algorithm. Finally the appointment of a Data Monitoring Committee ( DMC) and Independent Statistical Center is central to the strategy.
Operational Bias
Another key aspect is demonstrating the avoidance of operational bias, whereby auditable evidence must be provided that the SSR is based only on the pre-specified decision rule; that firewalls are in place to protect unblinded analyses and that the sponsor is not involved in the ISC and DMC interactions in any way.
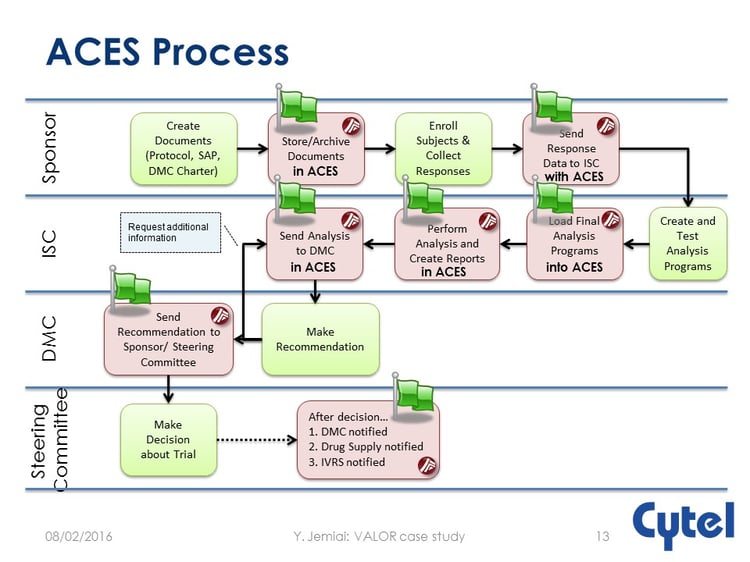
Here, Cytel’s ACES tool comes into its own, providing a secure environment specifically designed to handle DMC interactions.

The process is illustrated below.
To download Jemiai's slides click below.