Innovative Phase 3 Adaptive Enrichment Design in Oncology
 At a recent Pfizer/ Cytel seminar on rare disease and oncology development, Cytel’s Lingyun Liu presented innovative work on a patient enrichment design. In this blog, we share some design and operational considerations. This approach can help mitigate against underpowering of a clinical trial where there is uncertainty and heterogeneity of treatment effect among subpopulations.
At a recent Pfizer/ Cytel seminar on rare disease and oncology development, Cytel’s Lingyun Liu presented innovative work on a patient enrichment design. In this blog, we share some design and operational considerations. This approach can help mitigate against underpowering of a clinical trial where there is uncertainty and heterogeneity of treatment effect among subpopulations.
Background
( Please note this example is for illustrative purposes only.)
The background to the design is a compound in development for a rare and aggressive cancer. The incidence is low and with a very poor 5-year survival rate the current standard therapy offers limited treatment options and modest benefits.
The new agent ( Drug B) is an add-on to an existing treatment which we will call Drug A. No complete responses have so far been observed for such patients with Drug A. Drug B targets a different pathway. It is understood that targeting both these pathways concurrently is more effective than targeting either pathway on its own. In addition, the non-overlapping toxicity profile of the two drugs allows for prolonged dosing in responding patients.
Clinical Development Status
In this example, a Phase 1b/ 2 study has already been conducted and interim efficacy data shows a reduction in radiographic tumor volume with some patients presenting durable and complete responses. Within the population, there are two subgroups of patients -that we will call S+ and S based on a clinical marker, with better responses observed in patients in subgroup S+ . Given the rareness of the indication and the available safety and efficacy data, a confirmatory clinical study is planned.
Challenges
The clinical trial design for the confirmatory clinical trial needs to take into account a number of key factors:
- There is uncertainty about the treatment effect of drug B in combination with drug A compared with drug A as a single agent
- S is considered to be a more aggressive form of cancer and therefore the treatment response between the two groups S and S+ may be different. This heterogeneity in response has indeed already been observed in the ongoing Phase 1b/2 study.
- This uncertainty in treatment effect puts the study at risk of being underpowered.
The Solution
- Cytel Consultant Statisticians proposed an innovative adaptive enrichment design which uses fewer patients, preserves type I error and protects the power from deteriorating in case of the uncertainty of the treatment effect and the heterogeneity of treatment effects between the subpopulations
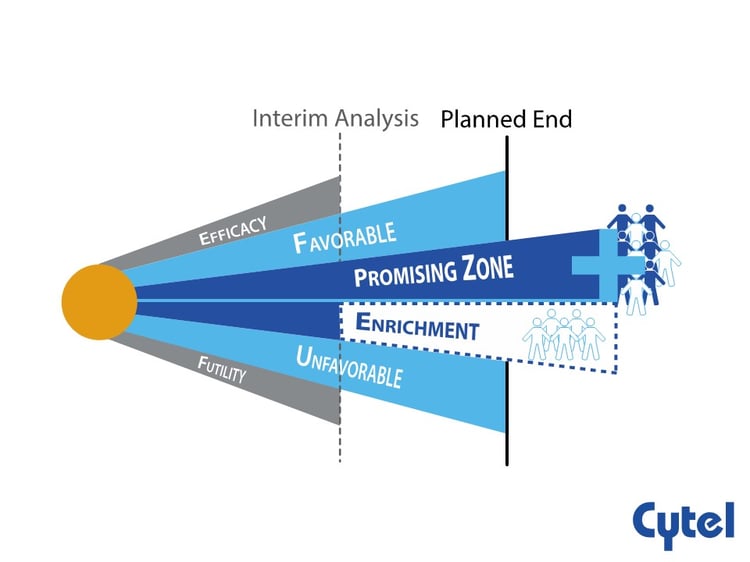
- At the time of interim analysis, the interim result will be classified. into favorable, unfavorable, promising and ‘enrichment’ zones based on conditional power.
- The sample size and Progression Free Survival events will be unchanged in the favorable and unfavorable zones, and will be increased in the promising zone to enhance the drug’s chances of demonstrating a benefit in a broader population, should one exist.
- Depending on the conditional power at the interim analysis, in the ‘enrichment’ zone, there is the possibility to recruit patients entirely from the potentially more responsive subpopulation ( S +) after interim analysis .
Conclusion
Such an adaptive enrichment design can help mitigate against underpowering of a clinical trial in a situation where there is uncertainty and heterogeneity of treatment effect among subpopulations and supports personalized medicine efforts.
Related reading:
2 methods for evaluating biomarker subpopulations in adaptive enrichment designs
Operational and regulatory considerations in a promising zone trial

