Patient Recruitment Feasibility: Would you bet $12 million dollars on it?
Two insightful papers from Applied Clinical Trials should be of interest to many clinical trial planners. The first by Kenneth Getz describes the problem of enrollment performance, while the second by Matthew Kibby proposes a potential solution.
The Facts
Getz reports a study providing recent estimates of industry-wide rates of enrollment delays [1]. In 2012, the Tufts Center for the Study of Drug Development (CSDD) requested data from 10 pharmaceutical companies and two CROs. The combined database covered nearly 16,000 investigative sites involved in 151 clinical trials from years 2008-2010. A few significant findings are worth highlighting:
- 11% of active sites failed to enroll a single patient.
- 41% of active sites failed to reach the target number of patients.
- One in six studies took more than twice as long as originally planned.
Getz advises that, “improved planning and forecasting gives sponsors and CROs greater confidence in study timelines…and provides a better basis to understand factors that most impact variance from planned performance.”
The Formula for Success
In light of the problems described by Getz, Kibby offers a relatively simple formula that lays the “foundation for establishing standard recruitment feasibility practices” [2]:
Enrollments = Rate * Time * Sites
- Enrollments = the number of randomized patients needed
- Rate = the number of patients per site per month
- Time = the enrollment time period in months
- Sites = the number of sites actively randomizing patients
When Enrollments, Time, and Sites are known, one can re-arrange the equation to solve for the required value of Rate.
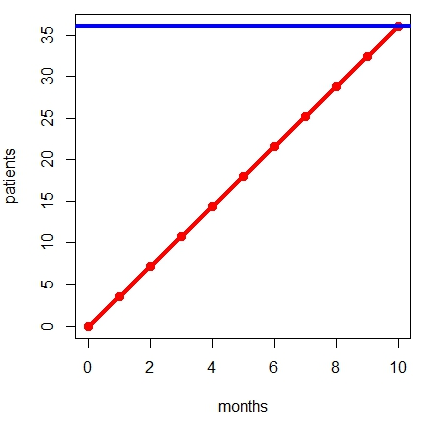
Kibby gives the following example. Suppose we need to recruit 1,800 participants across 50 sites in 10 months. The figure illustrates this example for a single site: On average, each of the 50 sites would need to target 36 patients (blue line), and therefore need 3.6 patients per month (red line).
Once this required recruitment rate is calculated, one can begin to assess how realistic it is. One might ask, for example:
- Can sites in every country accomplish this?
- Will each country be recruiting for all 10 months?
- How realistic is this randomization rate for each country?
Kibby subsequently poses the critical question: “…would you bet $12 million dollars on it?”
To justify potentially costly decisions in enrollment planning, one requires accurate assessments of the risks and rewards for various strategies, as well as their potential probability of success. While Kibby’s formula provides a starting point for such assessments, Getz’s findings remind us of their urgency.
Related Items of Interest
[1] Getz, KA. (2012). Enrollment Performance: Weighing the "Facts". Applied Clinical Trials, 21(5), p.24.
[2] Kibby M. (2011). Patient Recruitment Feasibility. Applied Clinical Trials, 20(6), p.80.
[3] How to Plan Interim Looks in Adaptive Clinical Trials: 3 Strategies
[5] Data-Driven Trial Planning: An Interview with Pfizer's Chris Conklin


