Key Design Considerations for Platform Trials
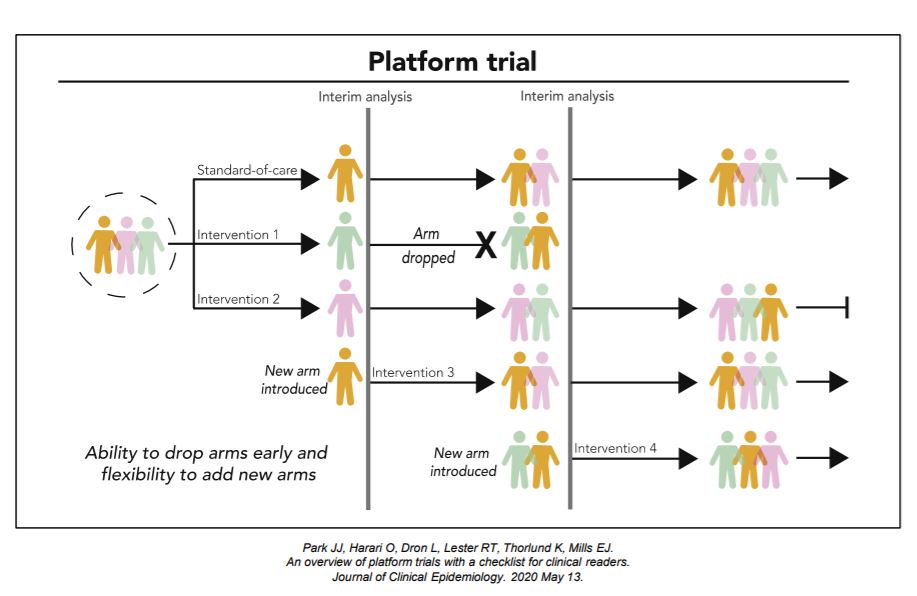
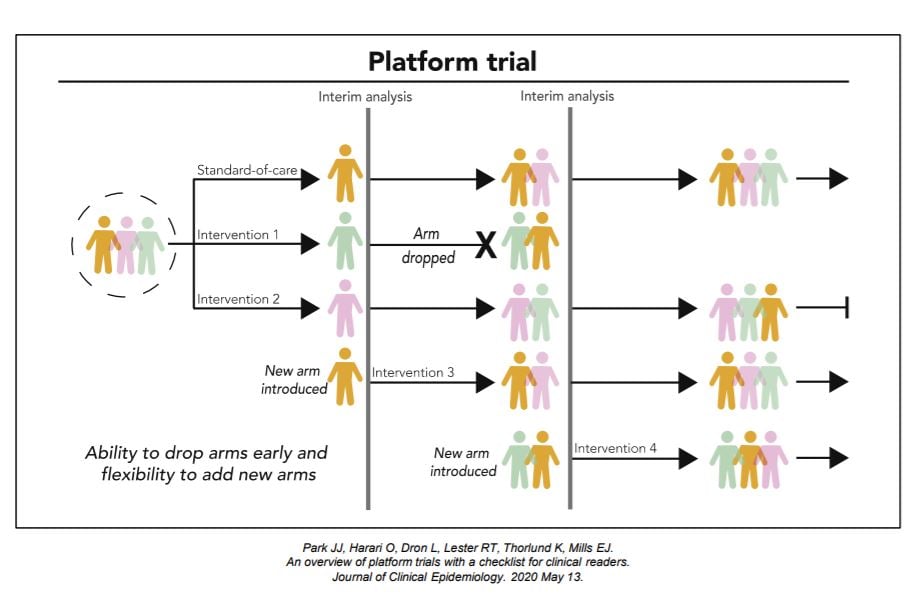
Platform trials are a new type of clinical trials where multiple interventions can be evaluated simultaneously against a common control group within a single master protocol. Platform trial designs are an extension of adaptive trial designs that are sometimes referred to as a multi-arm, multi-stage (MAMS) design, as multiple interventions (‘‘multi-arm’’) undergoing multiple interim evaluations (‘‘multi-stage’’) are part of the design features.
This autumn Cytel has been holding a number of webinars on Platform Trials, ranging from a discussion with Cyrus Mehta on statistical innovations to incentivize more sponsors to consider platform trials, to next week's event with Jason Connor (Confluence) on the use of Bayesian methods for these innovative trial designs.
In a recent webinar Jay Park, Director of Trials Research for Cytel in Canada, presented a webinar to review the concept of platform trials and discuss important design considerations for platform trials. Jay is the author of several leading papers on Platform Trials, including one in CA: A Cancer Journal for Clinicians, the journal with the world's highest impact factor. He has also produced a complimentary primer on the subject which you can download here.
Continue reading this blog to get a summary of his talk. Click the button to access the on demand webinar.
Background
Master protocols refer to a single overarching protocol developed to evaluate multiple hypotheses and the general goals are improving efficiency and establishing uniformity through standardization of procedures in the development and evaluation of different interventions. They are often classified as basket trials, umbrella trials and platform trials. This webinar focuses on platform trials, and to learn more about Basket and Umbrella Trials, you can watch a Cytel webinar by Jay here.
Cytel had conducted a landscape analysis that found 83 master protocols registered as on July 8th, 2019, of which 16 were platform trials. The COVID-19 Pandemic has further accelerated the acceptance of master protocols and platform trials with the I-SPY Trial[1] and WHO's SOLIDARITY Trial[2] paving the way. Cytel also led the implementation of the TOGETHER Trial to ensure more research towards early stages of COVID-19 in developing countries.
Adaptive Platform Trial
An Adaptive Platform Trial is a new type of clinical trial where multiple interventions can be evaluated simultaneously against a common control group with flexibilities of allowing new interventions to be added through the trial. It uses master protocol to outline standardized operating procedures and clinical trial evaluation plans. Adaptive platform trials are designed to be perpetual in nature and allow for hypotheses to be updated over time.
Platform Trials Vs Other Clinical Trials
A substantial number of trial designs now exist, but the majority of clinical trials can be categorized into three buckets i.e. fixed trial design, adaptive trial design or group sequential, and platform trial designs. In non-platform clinical trials, whether they are planned with fixed trial or adaptive trial designs, trials are not designed to add new interventions after the clinical trials start. Even if they are well-designed and -executed, the scope of clinical evaluation can be limited in these trials. For example, in a 2-arm clinical trial with most efficient adaptive trial designs, the trial can only answer whether a single intervention can offer benefits over existing standard-of-care or placebo for a given disease.
Multi-arm clinical trials are less intervention-dependent, but they face similar challenges since the scope of clinical evaluation is still limited to the interventions that get picked at the start of the trial. This becomes potentially problematic if the external scientific discovery outpaces the planned completion of the trial.
The intervention-agnostic approaches in Platform Trials on the other hand, allows us to answer the question of what the best treatment option is for a given disease in a single perpetual trial by allowing interventions to enter and leave at different time points.
Important Design Considerations for Platform Trials
Each platform trial has its own goals and subsequently, design features. For this kind of trials, it is important to consider how interventions are compared and evaluated throughout and how new interventions are introduced. For intervention comparisons, it is important to consider what the primary analysis is, what and how many interventions are active simultaneously, and allocation between different arms. Interim evaluation considerations should include the number and timing of interim evaluations and outcomes and statistical rules used to drop interventions. New interventions are usually introduced based on scientific merits, so consideration of these merits is important, together with the timing and mechanisms in which new interventions are added.
To get a better understanding of each of these key considerations along with illustrations, watch the on demand webinar by clicking the button.
Given their perpetual and intervention-agnostic nature, adaptive platform trials represent an important turning point in clinical research where we can achieve research that is not only more statistically efficient but also more patient centered. For further details, please refer to our recently published article on platform trials on JCE – Journal of Clinical Epidemiology - Park et al., 2020 “An overview of platform trials with a checklist for clinical readers”
References:
[1] I-SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer (I-SPY)
[2] “Solidarity” clinical trial for COVID-19 treatments
About Jay Park
 Jay is a Director of Trials Research for Cytel Canada Health. Inc based in Vancouver, BC. He recently joined the company in December 2019 when MTEK Sciences was acquired by Cytel. His research interests include application of master protocols, adaptive trial designs, and real-world analytics to global health. He currently teaches a course on adaptive trial designs and master protocols at McMaster University and is working on a book, “Introduction to Adaptive Trial Designs and Master Protocols” that will be published by Cambridge University Press in 2021.
Jay is a Director of Trials Research for Cytel Canada Health. Inc based in Vancouver, BC. He recently joined the company in December 2019 when MTEK Sciences was acquired by Cytel. His research interests include application of master protocols, adaptive trial designs, and real-world analytics to global health. He currently teaches a course on adaptive trial designs and master protocols at McMaster University and is working on a book, “Introduction to Adaptive Trial Designs and Master Protocols” that will be published by Cambridge University Press in 2021.


