Since 1953, when the discovery of the structure of DNA was made, we have seen great advancements in genomics. Particularly, in the last few years, the industry has seen a rapid rate of adoption in biomarkers and how they can be used to improve biomedical interventions. Trial investigators have been showing interest in biomarker-guided trials such as basket trials and umbrella trials, developed under the master protocol framework. As a result, we have been seeing a rapid rate of adoption of these innovative trial methods.
In our previous blog, we spoke with Jay Park, Director, Cytel, about the concept of master protocols, their importance and future growth potential. On March 19, Cytel conducted a webinar with Jay on “Key Design Considerations for Basket Trials and Umbrella Trials”. This webinar introduced two master protocol types and explored their extension to design in various contexts from the HIV epidemic in global health to expedited oncology trials. Continue reading for key highlights from the webinar .
Register now to get free access to webinar slides and recording.

Master protocols generally refer to a single overarching protocol developed to evaluate multiple hypotheses with general goals of improving efficiency and uniformity through standardized procedures. In 2017, the United States Food and Drug Administration (FDA) approved the first tumor-agnostic therapy and ever since then, the federal agency has been promoting innovative methods and master protocols, also referred to as complex innovative designs.
We have also witnessed a shift in the oncology landscape where increasing number of biomarker-targeted therapies are now being investigated and approved. This transition makes it even more necessary to understand the importance of biomarkers and how they are used to develop targeted therapies in clinical research.
In the webinar, Jay focuses on two of the categories of master protocols, namely, basket trials and umbrella trials.
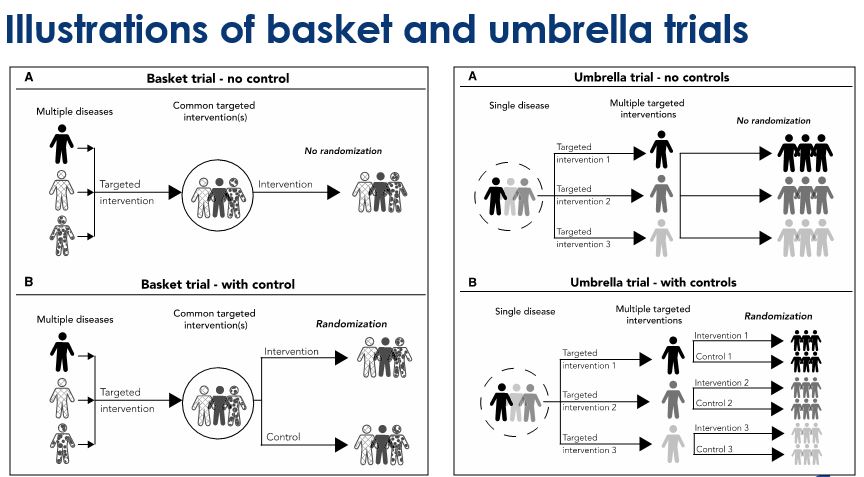
Basket trials - Refer to designs in which a targeted therapy is evaluated for multiple diseases that share common molecular alterations and/or other risk factors
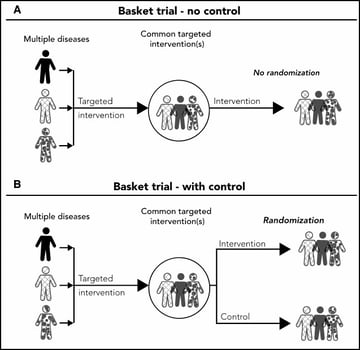
Umbrella trials - Refer to designs where multiple therapies are evaluated for a single disease that is stratified into multiple subgroups based on different molecular or other predictive risk factors
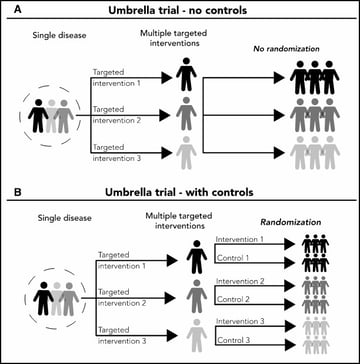
Despite the increasing numbers of basket and umbrella trials, they are still poorly understood. The purpose of this webinar was two-fold: firstly, to review the concept of master protocols and common characteristics of basket and umbrella trials; and secondly, to discuss important design considerations for these types of master protocols. Jay explains randomized and non-randomized, basket and umbrella trials through illustrations. And, in the second half, he gives us an insight on some of the key general considerations for designing these trials.
To know more about basket and umbrella trials, you may also refer to Jay’s recently published article in CA - A Cancer Journal for Clinicians: Park et al., 2020 “An Overview of Precision Oncology Basket and Umbrella Trials for Clinicians”
Register today to get access to the webinar recording and to download the presentation slides.

About Jay Park
 Jay is a Director for Cytel based in Vancouver, BC. He recently joined the company in December 2019 when MTEK Sciences was acquired by Cytel. His research interests include application of master protocols, adaptive trial designs, and real-world analytics to global health. He currently teaches a course on adaptive trial designs and master protocols at McMaster University and is working on a book, “Introduction to Adaptive Trial Designs and Master Protocols” that will be published by Cambridge University Press in 2021.
Jay is a Director for Cytel based in Vancouver, BC. He recently joined the company in December 2019 when MTEK Sciences was acquired by Cytel. His research interests include application of master protocols, adaptive trial designs, and real-world analytics to global health. He currently teaches a course on adaptive trial designs and master protocols at McMaster University and is working on a book, “Introduction to Adaptive Trial Designs and Master Protocols” that will be published by Cambridge University Press in 2021.





 Jay is a Director for Cytel based in Vancouver, BC. He recently joined the company in December 2019 when MTEK Sciences was acquired by Cytel. His research interests include application of master protocols, adaptive trial designs, and real-world analytics to global health. He currently teaches a course on adaptive trial designs and master protocols at McMaster University and is working on a book, “Introduction to Adaptive Trial Designs and Master Protocols” that will be published by Cambridge University Press in 2021.
Jay is a Director for Cytel based in Vancouver, BC. He recently joined the company in December 2019 when MTEK Sciences was acquired by Cytel. His research interests include application of master protocols, adaptive trial designs, and real-world analytics to global health. He currently teaches a course on adaptive trial designs and master protocols at McMaster University and is working on a book, “Introduction to Adaptive Trial Designs and Master Protocols” that will be published by Cambridge University Press in 2021.