Increasing Clinical Development Productivity Using Statistics and Cloud-Computing
The need for Re-imagining Clinical Trials: A recent survey conducted by Cytel found that only 42% of respondents reported using any complex or innovative clinical trial designs beyond the familiar group sequential approach. Although regulators respond quite favorably to such designs, sponsors have remained hesitant to use them.
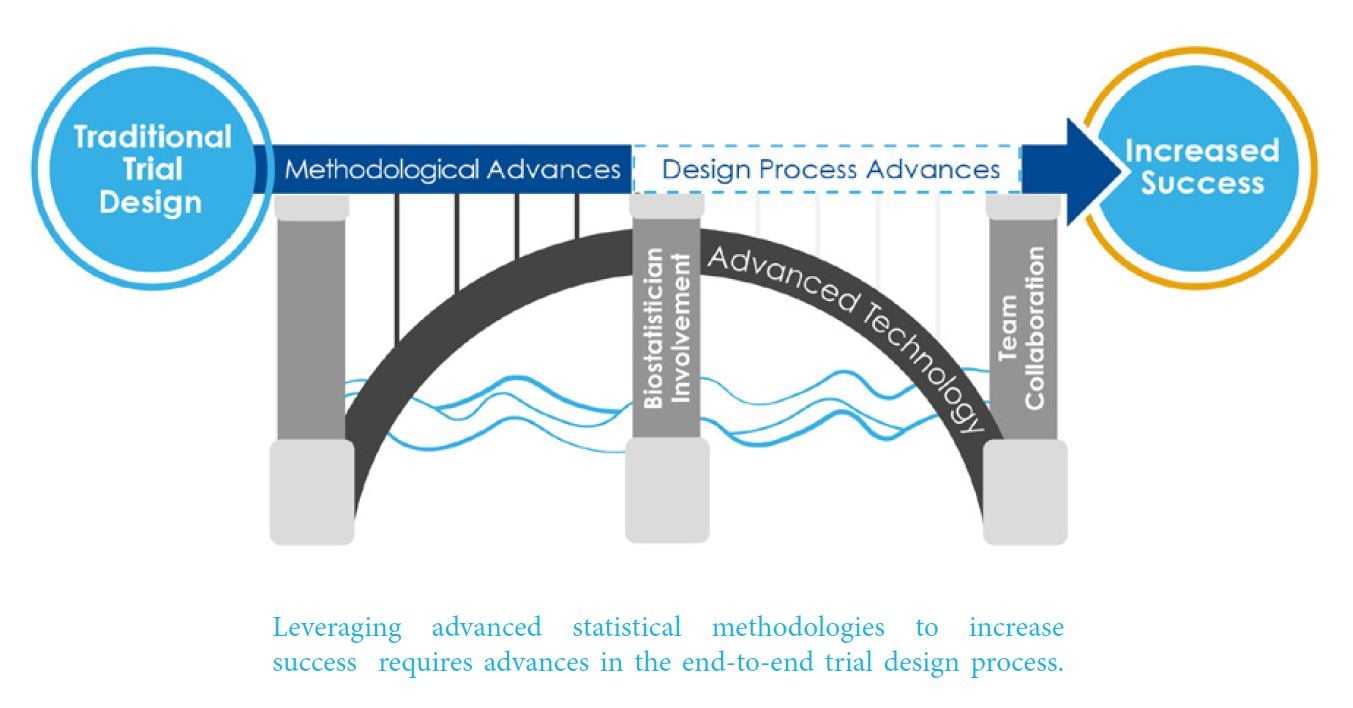
A combination of technological and process advances are necessary to overcome mechanisms that contribute to stagnating statistical innovation in clinical development. Cytel responded by creating this new whitepaper that provides a new strategic framework that can help Clinical Development teams leverage cloud-computing and begin to initiate process changes, necessary to increase development productivity by 10-20%.
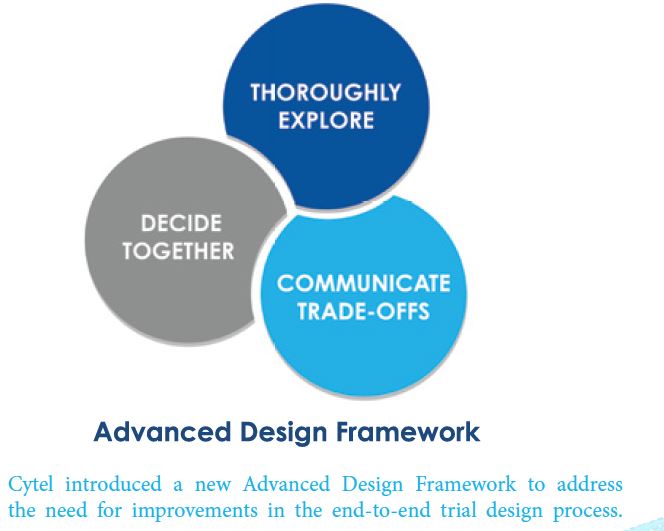
The new Advanced Design Framework has three steps: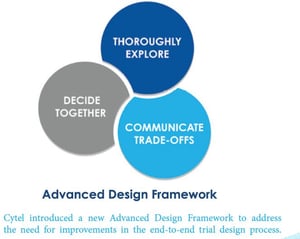
1. Thoroughly Explore the new statistical design spaces offered by cloud-computing
2. Decide Together using a quantitative evaluation approach that mitigates bias and generates data-driven collective-decision-making;
3. Communicate Tradeoffs in a way that translates statistical design into cogent evidence.
This new whitepaper will reveal:
- Why cloud-computing provides new opportunities and challenges to find globally optimized trial designs;
- Why trials have historically been chosen by satisficing rather than optimizing;
- How to overcome the communications challenges for nuanced decision-making;
- How to improve the speed and success of clinical trials.
For more information download: