Methods involving Group Sequential Designs are one of the earliest deviations from a traditional two-arm clinical trial with no interim looks at the data. They add incredible value to trials through their abilities to safeguard patients, reach positive conclusions early and keep trial designs simple and streamlined.
Sequential trials also help reduce costs and the number of patients involved, but finding a positive conclusion earlier is quite important too. In the drug development process, where patent lifetime is limited, reaching a decision six months or a year earlier is a big advantage. Sample Size Re-estimation is another key tool in the modern trial designer’s toolkit that proves to be useful. Continue reading this blog to learn how to use these methods and understand how they can improve trial design.
Group Sequential Designs Versus Fixed Sample Study
The scope of adaptive clinical trial design has grown in the last two decades. There are many new methods addressing novel issues such as, enrichment (focusing on a sub-population of patients), seamless trial designs that combine phase 2 and phase 3 trials, and multi-arm multi-stage trials.
Sequential analysis involves looking at the data as they come in and seeing when there is enough information to stop the trial. As part of the “Introduction to Complex Innovative Trial Designs” webinar series, Cytel conducted a webinar presented by Professor Christopher Jennison on Group Sequential Designs and Sample Size Re-estimation. Professor Jennison teaches Statistics at the University of Bath, UK, and has over 35 years of experience working in this area.
In the webinar, Professor Jennison focused on the design of Phase 3 trials. This phase is large and expensive, and more importantly, crucial in the process of drug approval. The challenge in a group sequential design is to reduce the length of these Phase 3 trials, thereby saving resources and reaching the conclusion sooner.
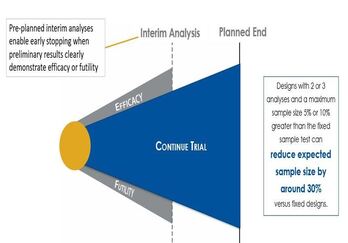
A fixed sample study is longer than a group sequential trial and does not involve any interim analysis. These pre-planned interim analyses in sequential designs enable early stopping when preliminary results clearly demonstrate efficacy or futility. Also, a group sequential design with two or three analyses and a small increase in sample over the fixed sample test can reduce expected sample size by around 30%.
Professor Jennison explains the theory behind group sequential method and how it works, through case studies. He uses an example of a trial for a cholesterol lowering drug and demonstrates how a fixed sample design and a group sequential design can be created on Cytel’s software East®.
Sample size re-estimation is another method to seek the same benefits of reduced sample size and an earlier conclusion. In a group sequential test, we set a large sample size and hope to stop early. In sample size re-estimation, we start with a small study and possibly increase the sample size post an interim analysis. The final set of data is then analyzed to make a conclusion.
Group Sequential Designs in East®
SEQUENTIAL is an East module for planning, simulating, monitoring and communicating group sequential clinical trial designs. It provides a wide variety of options for normal and binomial endpoints in superiority, non-inferiority, and equivalence studies. The software enables you to evaluate and compare the operating characteristics of different study designs on multiple information scales as well as graphically. You can plan sample size, time, and cost savings by building in efficacy and futility boundaries. You can also quantify your trial’s probability of success while remaining sensitive to deviations both analytically and via simulation.
To get more insights on these methods and see a demonstration of examples on our East® software, access the webinar recording and download the presentations slides.