Cytel's blog featuring the latest industry insights.

Use of the continual reassessment method (CRM) is safe, efficient, and flexible, according to a comprehensive review of 53 published Phase I trials from 2003-2013. The review, just published in the Journal of Clinical Oncology by leading researchers Iasonas and O'Quigley, challenges common misconceptions about model-based dose escalation designs.
In these published CRM trials, the average observed rate of dose-limiting toxicities (DLT) was relatively low (18%), while most patients (74%) were treated at or near the MTD. The trials were also efficient in terms of sample size and study duration.
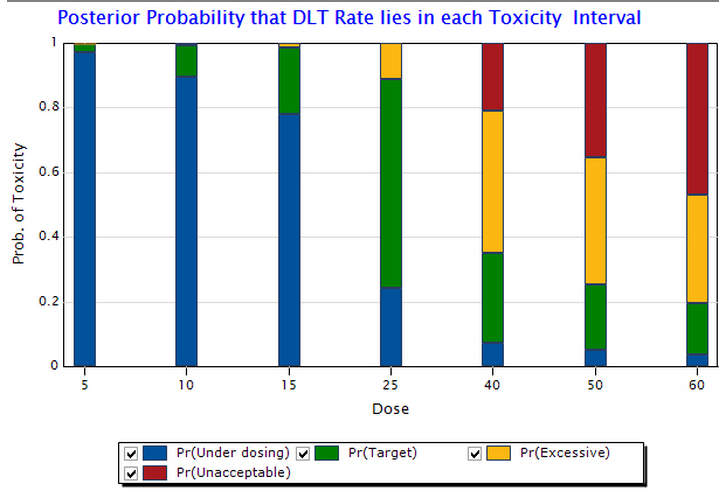
An often-ignored advantage of model-based designs is their flexibility. For example, probabilistic rules can be specified for overdose control, or for stopping the trial early. Concerns about aggressive dose escalation can be addressed simply by adjusting the cohort size, or not skipping doses. Iasonas and O'Quigley present detailed case studies of specific trials demonstrating these flexible options.
You can easily explore these characteristics of the CRM, and other model-based designs, in the new ESCALATE module in East 6.3, via simulations or analysis your own trial data.
Other Items of Interest
Adaptive Dose-Finding Studies: A Review of Model-Guided Phase 1 Clinical Trials
5 Reasons to Engage in Bayesian Dose-Escalation
Powering Oncology Trials for Success: Adaptive Designs in East
