
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

March 6, 2018
Photo by Dose Media on Unsplash By Charles Liu, Senior Product Manager, Cytel Several years ago, I was one of few...
Read article
June 4, 2015
Patient Recruitment Feasibility: Would you bet $12 million dollars on it?
Two insightful papers from Applied Clinical Trials should be of interest to many clinical trial planners. The first by...
Read article
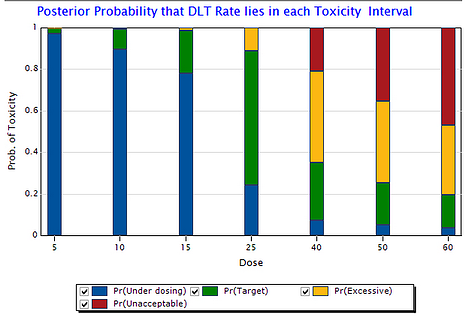
August 21, 2014
Bayesian Trial Designs are Safe, Efficient & Flexible: A Review of Published Phase 1 Studies
Use of the continual reassessment method (CRM) is safe, efficient, and flexible, according to a comprehensive review of...
Read article
July 15, 2014
Cytel Animation: Modern Dose Escalation Phase 1 Trials
Do you design or analyze Phase 1 dose escalation trials? Have you considered methods other than 3+3? This new Cytel...
Read article
June 5, 2014
Powering Oncology Trials for Success: Adaptive Designs in East
Charles Liu, PhD, Cytel Statistician and Product Manager In the US, cancer is the most common cause of death after...
Read article


