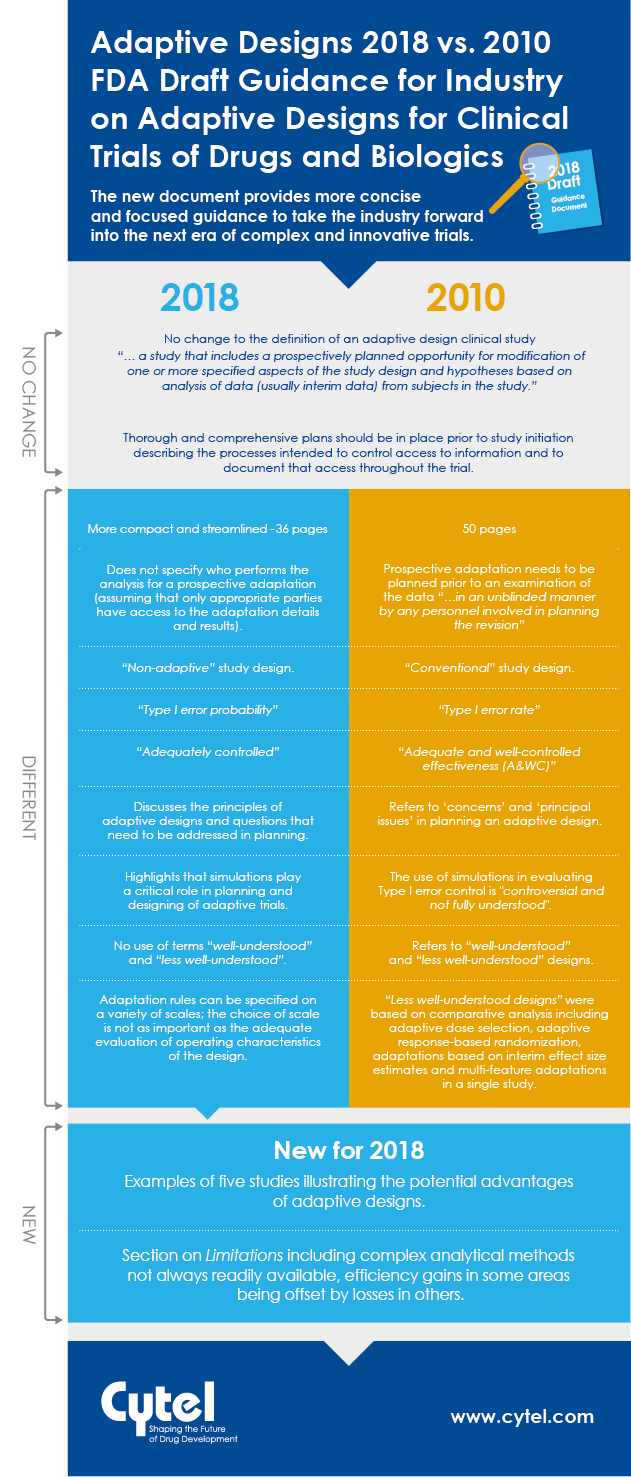
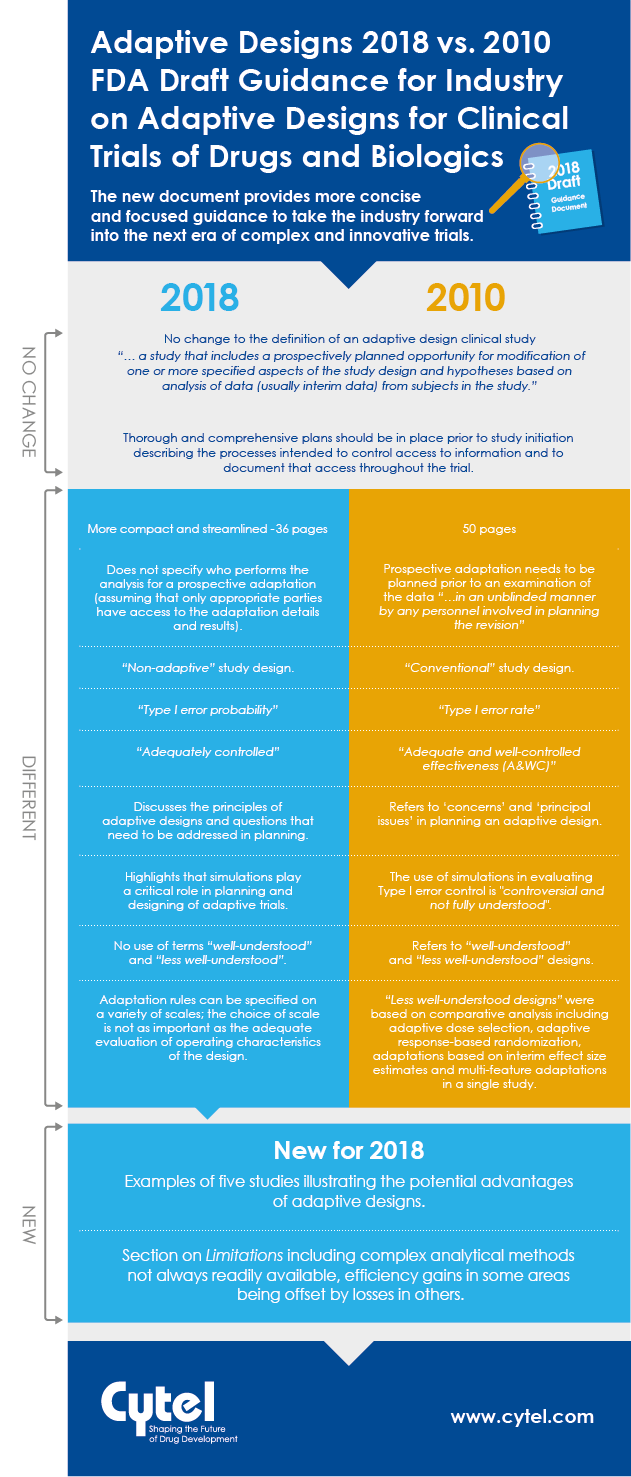
In September 2018, the FDA issued a new draft Guidance for Industry on Adaptive
Designs for Clinical Trials of Drugs and Biologics. This guidance replaces the
previously published 2010 draft. Natasa Rajicic, Principal, Strategic Consulting at Cytel reviewed the two documents to look for key differences and similarities between them, and identify any significant new elements introduced in the most recent material.
We are happy to share a handy infographic to illustrate how the two draft guidances stack up, and we are also delighted to share access to Natasa's full briefing note in this blog. The aspects she identifies, paint a consistent picture of a step forward in the FDA's thinking on adaptive designs. With a more significant body of adaptive design experience to draw on, the 2018 document provides more concise and focused guidance to take the industry forward into the next era of complex and innovative trials.
Click the button below to download the full briefing note and check out the infographic below.