
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

March 11, 2024
In 2023, rare diseases accounted for 30% of product pipeline under development, about half of which comprising...
Read article
January 17, 2024
Late-Stage Clinical Development Strategy: Trade-Offs and Decision-Making in the Confirmatory Setting
Despite accumulating learnings from early phases, several uncertainties remain to be addressed when designing pivotal...
Read article

January 10, 2024
Optimizing Early Clinical Development Strategy
A clinical development strategy is a comprehensive plan designed to establish the safety and efficacy of new...
Read article

December 29, 2023
The Top Most-Read Posts of 2023
What a year! Perspectives has explored a myriad of topics this year within clinical development — from adaptive trial...
Read article

December 22, 2023
Top Therapeutics Development Topics of 2023
Perspectives covers a wide range of topics within therapeutics development from advice on regulatory submission to...
Read article

December 20, 2023
Top Real-World Evidence and Real-World Data Topics of 2023
Perspectives covers a wide range of topics related to real-world evidence and real-world data, from overcoming health...
Read article

November 3, 2023
Reinforcement Learning: A Promising Tool for Predicting Optimal Treatment in Complex Diseases
Written by Fei Tang and Evie Merinopoulou Reinforcement Learning (RL), a crucial component of machine learning (ML),...
Read article

October 27, 2023
News from ESMO: Challenging the Status Quo of Early Phase Clinical Trial Design — Moving from “Why” to “How”
Written by Natalia Muehlemann, Vice President, Clinical Development; Martin Frenzel, Research Principal, Statistical...
Read article

September 22, 2023
Myth Busting: Master Protocol Edition
Interest and appetite for master protocols is growing as sponsors consider opportunities in various therapeutic areas...
Read article
January 25, 2023
Bayesian Approach in Oncology Trials
People think in Bayesian terms all the time: we use prior information and the evidence at hand to make decisions in our...
Read article

January 23, 2023
Design Considerations for Early Phase Trials of Immuno-oncology Drugs
Ever since the first immune checkpoint inhibitor was approved for market nearly twelve years ago, the industry has...
Read article
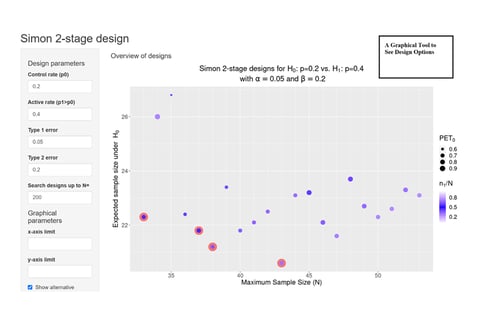
June 8, 2022
On Kappler's 'Graphical Comparison of Simon two-stage designs'
Clinical researchers, seeking to understand the statistical benefits of a common Phase 2 oncology design, now have a...
Read article

May 16, 2022
Cytel & ARCS Collaborate to Strengthen Early Phase Capabilities
A combination of industry and policy forces have recently changed the shape of Australia’s R&D sector, making it a...
Read article

April 7, 2022
Bridging the gap between oncology clinical trials and real-world data: evidence on replicability of efficacy results using German claims data
Randomized control trials (RCTs) are the gold standard for estimating the efficacy of a treatment. They allow us to...
Read article

March 18, 2022
The Fundamentals of Real World Evidence in Oncology Drug Development
Real world evidence (RWE) provides a large and growing source of insights into drug uptake and safety. It is...
Read article

March 8, 2022
How to conduct better time-to-event analysis with delayed treatment effects
The issue of delayed treatment effects in immuno-oncology was demonstrated during a FDA-Industry sponsored workshop...
Read article

November 30, 2021
The FDA “Real-Time Oncology Review” Process
The FDA “Real-Time Oncology Review (RTOR)”[1] is an “FDA project started in 2018 to facilitate earlier submission of...
Read article

October 22, 2021
The Benefits of Using Basket Studies in Oncology
Currently, there are many treatment options for Cancer such as, Immunotherapy, Radiation Therapy, Chemotherapy etc. If...
Read article

July 1, 2021
Myths surrounding the use of synthetic control arms in clinical trials: Part 4
Synthetic control arms (SCAs) are best suited for situations when a single arm trial is run in a patient population...
Read article

April 7, 2021
Career Perspectives: Interview with Neha Sati
Scientists at Cytel recently published a paper in the Journal of the American Medical Association (JAMA). Among the...
Read article
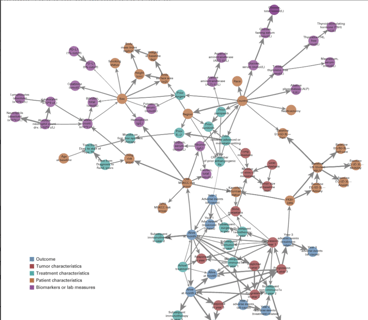
April 2, 2021
Using Bayesian Networks to Predict Survival Outcomes: New Case Study
Earlier this month, my colleagues at Cytel Canada published a paper in JCO Clinical Cancer Informatics, offering a...
Read article

March 11, 2021
Data and analysis in Modern Oncology Clinical Development
In the recent years, Oncology trials are seeing a technological shift that is expected to make them faster and more...
Read article

March 5, 2021
Single Arm Multi-Stage Phase 2 Cancer Trials
Early stage Phase 2 clinical trials are often designed as multi-stage single arm trials, which quickly identify...
Read article

February 12, 2021
Leveraging Synthetic and External Control Arms Using Bayesian Methods
In recent times, Single arm trials are being increasingly used to assess new treatment interventions. They establish...
Read article

February 9, 2021
New Meta-Analysis in JAMA Uses Novel Quantitative Techniques to Demonstrate Baseline Characteristics Informing Response to Common Therapy for Kidney Cancer
Recent years have witnessed improving survival outcomes for those struggling with a range of common kidney cancers....
Read article

November 24, 2020
Cytel and Ingress Health at Virtual ISPOR Europe 2020
Virtual ISPOR 2020, held November 16 to 19, presented new opportunities for scientific interaction amongst HEOR...
Read article

November 16, 2020
Bayesian Methods for Multiple Cohort Expansion (MuCE) designs
MUCE is a Bayesian solution for cohort expansion trials where multiple dose(s) and multiple indication(s) are tested in...
Read article

November 12, 2020
Join Cytel and Ingress Health at Virtual ISPOR Europe 2020
Cytel and Ingress Health (now a Cytel company) will be contributing to a range of events at Virtual ISPOR EUROPE 2020,...
Read article

November 5, 2020
Role of RWA in Transforming Oncological Research
In oncology, many manufacturers go into niche indications, where there are very specific tumors, and then they opt for...
Read article

October 22, 2020
An Interview with Bart Heeg (Part 2): New Trends in HEOR
In this two-part blog series, we interview Bart Heeg, Vice President HEOR and Founder at Ingress Health (A Cytel...
Read article

October 20, 2020
Interview with Thomas Wilke: Health Economics/World Evidence Studies
In this interview with Thomas Wilke, Principal Scientist at Ingress-Health (a Cytel company), we talk to him about his...
Read article

October 19, 2020
The Uniqueness of COVID-19 Data Challenges; The COVID-19 trial tracker
COVID-19 has created extreme uncertainties -- a dearth of historical information combined with the need for safety,...
Read article

October 14, 2020
The Increasing Importance of Health Economics
A credible evidence base is needed to support and document the economic value of new technologies and therapeutic...
Read article

September 10, 2020
Does your Trial need a Synthetic Control Arm?
Single arm trials are emerging as an accepted way of assessing a new treatment intervention. They establish clinical...
Read article

August 7, 2020
Creating a Synthetic Control from Your Natural History Study
Recently a biotech approached Cytel for support with a Phase 2 Study in oncology. Regulators had requested a natural...
Read article

August 5, 2020
Design and Data Considerations from Cardiovascular Pilot Investigation
Cytel is conducting two pilot projects on head-to-head comparisons using real world data. These projects in oncology...
Read article

July 30, 2020
Cytel Scientists Call for a “Statistician-First Workflow” to Optimize Drug Development
A new peer-reviewed article co-authored by several Cytel scientists re-examines the way in which adaptive trials are...
Read article

July 21, 2020
Head to Head Comparisons Using Real World Data
Cytel is conducting a webinar series that focuses on target trial emulation and causal inference approaches using real...
Read article

July 15, 2020
Three Reasons Why Oncology Trials Need Clear Estimands
Unlike many therapeutic areas, oncology benefits from having standardized endpoints like overall survival and...
Read article

July 7, 2020
Overcoming Clinical Development Challenges in Oncology with Innovative
Having its roots in the seminar rooms of the Dana Farber Cancer Institute, Cytel has a long record of establishing new...
Read article

June 24, 2020
Webinar - Practical Model-based Approaches for Phase I Oncology Trials
Last week, Cytel conducted its third webinar in the new introductory webinar series on Complex Innovative Trial...
Read article

June 22, 2020
Webinar: Synthetic and External Controls in Clinical Trials
Cytel scientists recently published a new eBook on synthetic control arms and a new scientific primer for the more...
Read article

June 15, 2020
Significance of Bayesian Model-Based Approaches in Oncology Trials: An Interview with Dr. Satrajit Roychoudhury
Cytel conducted a webinar with Dr. Satrajit Roychoudhury, Senior Director, Statistical Research and Data Science...
Read article

June 8, 2020
New Primer and Ebook on Synthetic Control Arms
Cytel has recently published a new ebook on synthetic control arms, and a new scientific primer as well.
Read article

May 12, 2020
Oncology Trial Design & Development Webinar Series
In our previous blog, “Remote Working Arrangement – How to get it right?”, we talked about how the need for social...
Read article

May 5, 2020
Webinar: A Clinician’s Perspective on Cancer Drugs Development
Cytel's team of oncology trial design and advanced analytics experts are hosting a series of complimentary webinars...
Read article
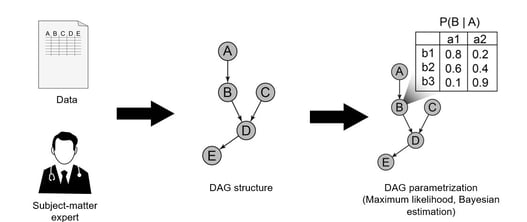
April 29, 2020
Webinar: Transparent Machine Learning in Oncology
In our previous blog, we spoke with Alind Gupta, who works as a Machine Learning Researcher at Cytel in Canada. The...
Read article

April 23, 2020
A Clinician’s Perspective on Cancer Drugs Development
Cytel is hosting a webinar, “A Clinician’s Perspective on Cancer Drugs Development”, on April 28, 2020. Our speaker,...
Read article

April 20, 2020
Interview with Alind Gupta: Transparent Machine Learning in Oncology
Cytel is hosting a webinar on Transparent Machine Learning in Oncology, on April 21, 2020. Our speaker, Alind Gupta,...
Read article
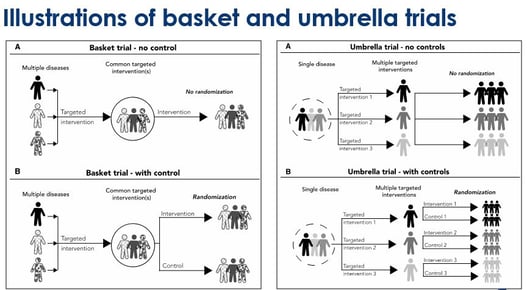
March 31, 2020
Key Design Thoughts for Basket Trials and Umbrella Trials by Jay Park
Since 1953, when the discovery of the structure of DNA was made, we have seen great advancements in genomics....
Read article

March 12, 2020
Interview with Jay Park: The present and future of Master Protocols
In September 2018, the FDA provided a draft guidance on master protocols reflecting an increased interest in these...
Read article
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article

November 5, 2019
Drug Development in Rare Diseases - Innovation in Statistical Thinking
Cytel is delighted to have Kannan Natarajan speaking at the “Complex Innovative Trial Design Symposium and East User...
Read article
October 10, 2019
The Challenges of Rare Diseases in Clinical Trials Symposium and Hands-on East Training
A disease is generally considered to be rare if it affects one patient per 200,000 people (1) and most rare diseases...
Read article

August 1, 2019
Predictive Biomarker Signature Characterization
The term biomarker signature describes the behavior of a set of biomarkers that define a signature to maximize the...
Read article

June 27, 2019
Handling the specialized data requirements in oncology clinical trials
By Nicolas Rouillé and Eric Henniger The right design and the right data ultimately leads to the right decisions, so...
Read article

March 26, 2019
How Patient-Reported Outcomes Improve Outcomes
At the Partnerships in Clinical Trials Conference in Barcelona in November 2018, Strategic Consultant Ursula Garczarek...
Read article

November 5, 2018
New publication addresses critical issues in ultra-orphan indications
Cytel biostatisticians Cyrus Mehta and Lingyun Liu, together with Charles Theuer, CEO of TRACON Pharmaceuticals have...
Read article

September 17, 2018
Novel immunotherapies lean on old methods
Immunotherapy has brought us many promises, most notably, of a future where humans are able to harness their body’s own...
Read article

September 7, 2018
Opportunities of FDA’s Innovative Trial Design Pilot Meeting Program
On August 29th 2018, the FDA announced (1) that it would be establishing a Complex Innovative Trial Design (CID) Pilot...
Read article

May 23, 2018
Addressing Critical Unmet Oncology Needs in the Era of Precision Medicine
Our Industry Voices series showcases our clients’ innovative work and breakthrough therapeutics in oncologic...
Read article

May 16, 2018
Rewriting the oncology textbook with cell-based immunotherapies
Our Industry Voices series showcases our clients’ innovative work and breakthrough therapeutics in oncologic...
Read article

May 9, 2018
Innovative Oncology Trial Designs in Practice
As we prepare to head to ASCO in under a month's time, we are pleased to share a new ebook that showcases some key...
Read article

April 25, 2018
Overcoming Data Management Challenges in Immuno-Oncology Trials
Data management is an essential building block for successful Immuno-Oncology (I-O) trials. At the Immuno-Oncology...
Read article

February 27, 2018
Congrats to Lipopharma and CLINGLIO Consortium on Recent Grant Award
We extend our congratulations to Lipopharma and the CLINGLIO project consortium on their recent 6,15M€ grant award by...
Read article

October 27, 2017
Design Concept for Confirmatory Basket Trial Interview with Bob Beckman: Part 2
In this blog, we share the second part of our interview with Bob Beckman, about a design concept for a confirmatory...
Read article

September 11, 2017
Design Concept for Basket Trials: Interview with Bob Beckman
At the East User Group meeting (EUGM) on 25th and 26th October, we will welcome a number of renowned industry speakers...
Read article

July 26, 2017
Are Adaptive Designs the Answer to Oncology Development Success?
Sadly, clinical development of anti-cancer therapeutics faces particularly high rates of failure, even in the context...
Read article
April 28, 2017
Case Study: Redesigning a Pragmatic Trial in Oncology
In this blog we share a case study in which our statistical consulting team helped a client redesign an oncology...
Read article

March 10, 2017
Flexible approaches to Biosimilars Development
At the recent Biosimilars Summit in Philadelphia, Cytel's Pantelis Vlachos presented on statistical challenges and...
Read article

March 2, 2017
Case Study: Bayesian Decision-Making in a Phase 3 Oncology Design
We continue our case study series with this example of a Phase 3 design that uses Bayesian decision making combined...
Read article

February 9, 2017
Inside an Oncology Statistician's Toolkit
In this blog, Adam Hamm, PhD, Director Biostatistics at Cytel shares some of the most important knowledge he uses in...
Read article
December 2, 2016
Innovative Phase 3 Adaptive Enrichment Design in Oncology
At a recent Pfizer/ Cytel seminar on rare disease and oncology development, Cytel’s Lingyun Liu presented innovative...
Read article

September 27, 2016
Practical Challenges of the LUNG-MAP study
The Lung-MAP trial is an innovative biomarker driven 'precision medicine' study which evaluates five novel agents for...
Read article
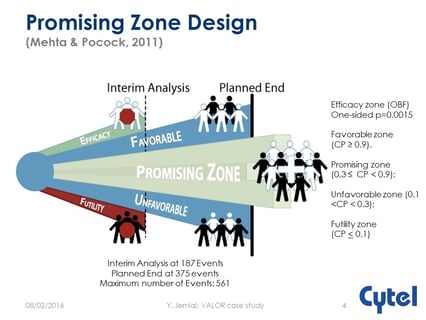
August 9, 2016
Operational and regulatory considerations in a promising zone trial
At the recent JSM Meeting, Cytel’s Yannis Jemiai presented the case study of the VALOR trial which used a promising...
Read article

July 21, 2016
Webinar Replay: Single and Double Agent Dose Escalation Designs
Did you miss our webinar on Single and Dual Agent Dose escalation designs earlier in the year? In this blog we have...
Read article
April 26, 2016
Overcoming Data Management Challenges in Oncology Studies
In this blog we’ll highlight some unique challenges that are encountered from a Data Management perspective when...
Read article

April 22, 2016
Dual Agent Dose Escalation Designs
FDA draft guidance on “Co development of two or more unmarketed investigational drugs for use in combination” notes...
Read article

April 13, 2016
HTAs: Adjusting Overall Survival for Treatment Switch
We continue our series of blogs covering the expert presentations from the EAST User Group Meeting. Consultant Claire...
Read article
April 9, 2015
Relative Clinical Efficiency and Phase 2 Biomarker Studies
Last year. Nature Reviews Drug Discovery asked the FDA’s Tatiana Prowell (Hematology & Oncology Products Division)...
Read article
November 4, 2014
New Exploratory Trial Method Translates into Better Financial Strategy
A key stage of exploratory drug development is implementing a proof-of-concept study to demonstrate the safety of a...
Read article
October 28, 2014
Adaptive Dose Finding Using Toxicity Probability Intervals
Phase 1 oncology trials typically use either rule-based methods or model-based methods to determine the most acceptable...
Read article
September 18, 2014
5 times ‘Keep it Simple’ May Be Bad Advice for Clinical Designers
When designing clinical trials, many trial designers are advised to keep the trial simple. Prima facie, the keep it...
Read article
July 10, 2014
Designs for Biomarker Driven Population Enrichment in Oncology
Complexities with identifying suitable test populations in oncology studies contribute significantly to the 60%...
Read article
July 8, 2014
5 Reasons to Invest in Adaptive Designs for Population Enrichment
The above graphic is from Cyrus Mehta's slides on 'Adaptive Population Enrichment for Oncology Trials with Time to...
Read article
June 26, 2014
Adaptive Designs for Precision Medicine: A Look at Pfizer
The rise of biomarker based treatments in oncology has meant a reconceptualization of what constitutes a particular...
Read article
June 10, 2014
Cytel Weighs in on Strategies for Oncology Development
The FDA’s Tatiana Prowell (Breast Cancer Scientific Lead in the Office of Hematology & Oncology Products) recently gave...
Read article
June 5, 2014
Powering Oncology Trials for Success: Adaptive Designs in East
Charles Liu, PhD, Cytel Statistician and Product Manager In the US, cancer is the most common cause of death after...
Read article
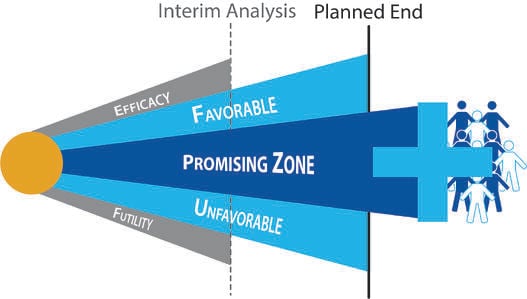
May 13, 2014
De-Risking Drug Development using Adaptive Design
The VALOR trial recently applied a promising zone design to a Phase 3 evaluation of Vosaroxin, a candidate for the...
Read article
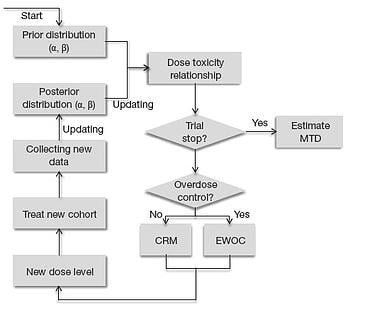
May 8, 2014
5 Reasons to Invest in Bayesian Dose-Escalation Methods
( Editor's note: This post has been refreshed in December 2016) Model based algorithms for Phase I dose-escalation have...
Read article

April 21, 2014
StatXact 25th Anniversary: A Horizon for the Stars
The core methodological problem that would eventually spur the development of Cytel’s StatXact software was first posed...
Read article


