
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

September 22, 2020
Research Scientists, Thomas Wilke and Sabrina Mueller recently published a manuscript on “Diabetes-Related...
Read article

April 2, 2020
Remote Working Arrangement – How to get it right?
On March 16, the World Health Organization (WHO) Director-General, Dr. Tedros Adhanom Ghebreyesus, in his media...
Read article

February 28, 2019
Statistical Approaches to Overcome Challenges in Rare Disease Development
In honor of Rare Disease Day 2019 we share a new Cytel podcast featuring Cytel Strategic Consultant Ursula Garczarek...
Read article

February 21, 2019
Podcast with Ursula Garczarek on The Effective Statistician
In 2018, Cytel ran a qualitative survey among biostatisticians and programmers on trends in data science and...
Read article

January 30, 2019
Career Perspectives: Interview with Tina Checchio, Associate Director, Quantitative Pharmacology & Pharmacometrics
QPP remains at the heart of model based drug development. Short for Quantitative Pharmacology & Pharmacometrics, it...
Read article
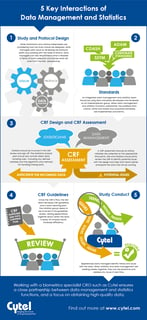
July 27, 2018
Infographic: 5 Key Interactions of Data Management and Statistics
In this blog, we share a new infographic based on this popular blog post illustrating some of the critical interactions...
Read article

June 19, 2018
The Importance of Standardization in Clinical Outsourcing
At the recent PCMG conference in Malta, Adrian Otte ( Independent Consultant, formerly VP Global Development Operations...
Read article

April 25, 2018
Overcoming Data Management Challenges in Immuno-Oncology Trials
Data management is an essential building block for successful Immuno-Oncology (I-O) trials. At the Immuno-Oncology...
Read article

March 14, 2018
What makes a Successful FSP Partnership Tick?
Photo by Agê Barros on Unsplash by Natalie Fforde, Senior Director of FSP Services at Cytel With effective use of...
Read article

November 22, 2017
Career Perspectives: Interview with Makarand Deshmukh, Senior Clinical Data Analyst
Cytel offers a full range of clinical data management services and the team of experts is spread across the globe. In...
Read article

November 15, 2017
Creating Efficiencies in the Vendor Qualification Process: A Proposal
Each year Halloran Consulting Group hosts‘CORE’ (Clinical Operations Retreat for Executives) as a forum for industry...
Read article

September 29, 2017
Career Perspectives: Interview with Namrata Deshpande, Senior Team Lead
Namrata Deshpande, Senior Team Lead will be participating in a round table discussion at the Women in Statistics event...
Read article

September 13, 2017
How can Novel Statistical Methods Tackle Antibiotic Resistance?
Antibiotic resistance is one of the greatest challenges facing human health today. We are excited to welcome Dr. Scott...
Read article

September 11, 2017
Design Concept for Basket Trials: Interview with Bob Beckman
At the East User Group meeting (EUGM) on 25th and 26th October, we will welcome a number of renowned industry speakers...
Read article

August 2, 2017
Case Study: Cross-study Efficiencies in Biometrics Outsourcing
As a biometrics -focused CRO, Cytel regularly works across a program of studies, providing data consistency, and...
Read article
July 19, 2017
Case Study: Seamless Independent Data Monitoring Committee Support
With adaptive and innovative trial designs on the rise, operational implementation of interim analyses, including...
Read article

July 13, 2017
Creating Data Visualizations with R and Shiny
By Tejasweeni Rajput It’s been known for centuries that a picture can tell a thousand words. In an era of new...
Read article
June 28, 2017
Under wraps: the importance of patient privacy
About the Author: Manjusha Gode has over 28 years' IT experience spanning delivery Management, quality management,...
Read article

March 20, 2017
The Role of the Independent Randomization Center
In the randomized clinical trial (RCT), the process of deciding the randomization method and implementing is critically...
Read article

March 15, 2017
The Data Management Plan Takes Center Stage- why is it so important?
A precise and thorough approach to planning is key for success in data management. The Data Management Plan (DMP) is a...
Read article

November 21, 2016
Infographic: 10 steps to consider before choosing an adaptive design
While adaptive designs can deliver significant benefits to clinical development- including ethical benefits for...
Read article
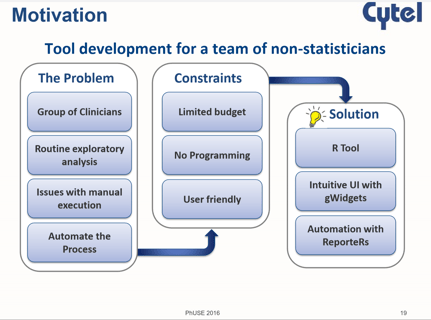
October 25, 2016
R Beyond Statistics
Use of R is a hot topic among statisticians and programmers in the pharmaceutical industry. At the recent PhUSE...
Read article
October 13, 2016
The evolving role of the modern statistical programmer
Statistical programmers play a key role in turning the data from clinical trials into knowledge and supporting the...
Read article
October 5, 2016
Challenges in Neuroscience Clinical Trials
While some progress has been made in terms of scientific development in Neuroscience and Neuropsychiatry indications,...
Read article
September 9, 2016
Case Study:Seamless Phase 2/3 Design in Rare Disease
Challenge: Our client, an emerging biotechnology company, was preparing for the next stage of development for their...
Read article

September 7, 2016
Overcoming challenges of 'Less Well Understood' Adaptive Designs
In the 2010 draft FDA ‘Guidance for Industry on Adaptive Design Clinical Trials for Drugs and Biologics', the agency...
Read article
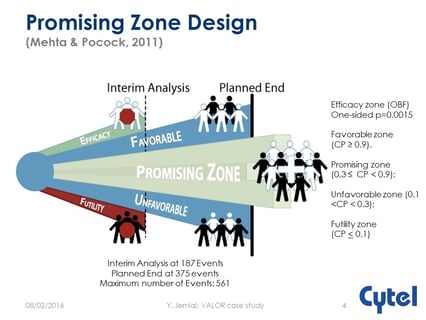
August 9, 2016
Operational and regulatory considerations in a promising zone trial
At the recent JSM Meeting, Cytel’s Yannis Jemiai presented the case study of the VALOR trial which used a promising...
Read article
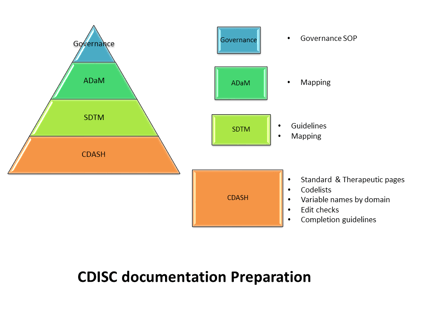
August 2, 2016
The CRO role in Data Standards Governance
Editor's note( this blog was refreshed in April 2018) As CDISC compliant submissions become increasingly expected,...
Read article
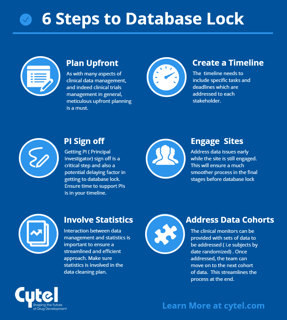
July 28, 2016
6 steps to timely database lock
To close a clinical database right the first time you have to begin with study start-up. Clearly, you can’t close a...
Read article

July 12, 2016
Key considerations in selecting an EDC system
How do you go about selecting the best Electronic Data Capture (EDC) system for your study? There is now a vast amount...
Read article

June 23, 2016
Getting the best out of your biometrics RFP
Vendor selection is a critical component of ensuring clinical trial success. A 2015 report (1) suggested that clinical...
Read article
June 14, 2016
Managing DMC analysis- an innovative programming solution
At Cytel, we are very often asked to get involved in DMCs ( Data Monitoring Committees) in a variety of capacities. Our...
Read article

May 12, 2016
Lost in Traceability- From SDTM to ADaM
Once upon a time Hansel and Gretel laid a trail of breadcrumbs which they followed to find their way back home. Their...
Read article
April 26, 2016
Overcoming Data Management Challenges in Oncology Studies
In this blog we’ll highlight some unique challenges that are encountered from a Data Management perspective when...
Read article

April 20, 2016
Handling CDM data integrations
During the course of any clinical trial, there are often data which, while collected electronically, are outside of the...
Read article

April 15, 2016
5 Key Interactions of Clinical DM and Statistics
It's critical for biostatistics and data management to be closely aligned and working effectively together. The...
Read article

February 26, 2016
Getting Technical: The evolving role of the Data Manager
Remember the early days of Electronic Data Capture? Those first systems, which were revolutionary for their time...
Read article
November 5, 2015
The 24 Hour Work Day
Oftentimes people perceive a tradeoff between speed and quality. The faster you do something the more likely you are to...
Read article

August 13, 2015
Do you really need a full service CRO? An exploration of strategic options
Full service or specialized? Full service or specialized? For many looking to hire a CRO, the answer is obvious....
Read article
June 5, 2015
Building Teams to Handle Unexpected Regulatory Agency Requests
Not long ago, one of our clients submitted Phase 2 and Phase 3 data for a new rare disease drug which had received...
Read article

May 28, 2015
How to Use Outsourcing to Reduce Clinical Development Risk
Risks in drug development range from taking the wrong drugs forward to Phase 3 to investing in a drug development...
Read article
April 21, 2015
Adaptive SSR for Small Sample Sizes?
“We shouldn’t use an adaptive design, our sample size is too small.” Most clinical trial planners have heard this line...
Read article
December 2, 2014
How to Incorporate New Technology into Your Clinical Development Strategy
During a recent DIA webinar on reinventing the clinical trial, Laurie Halloran (President of the Halloran Consulting...
Read article
October 7, 2014
You Own This!
Guest blogger Laurie Halloran is the President and CEO of Halloran Consulting Group, a management consulting firm for...
Read article

July 24, 2014
Data Management and Biostatistics III: Statistical Innovation in Clinical Data Management
This is the third post in a three part series in which we consider (i) improvements to trial quality that result from...
Read article

June 3, 2014
Data Management & Biostatistics II: Operational Benefits of Bundling
This is the second post in a three part series in which we consider (i) improvements to trial quality that result from...
Read article

May 22, 2014
Data Management & Biostatistics I: Improving Trial Quality
This is the first of a three part post in which we will consider (i) improvements to trial quality that result from...
Read article


