Predictions of Pharmacodynamic Responses in Ulcerative Colitis Patient
 The Population Approach Group in Europe (PAGE) represents a community with a shared interest in data analysis using the population approach. Each June, a meeting of the community is held at a different European location. At this year's meeting in Budapest, Hungary, Cytel's Director of Quantitative Pharmacology and Pharmacometrics, Cecilia Fosser, showcased innovative work on creating model based predictions of pharmacodynamic responses in ulcerative colitis patients. Fosser presented a poster Model based predictions of the PTG-100 pharmacodynamic responses in ulcerative colitis patients created in collaboration with colleagues from Protagonist Therapeutics.
The Population Approach Group in Europe (PAGE) represents a community with a shared interest in data analysis using the population approach. Each June, a meeting of the community is held at a different European location. At this year's meeting in Budapest, Hungary, Cytel's Director of Quantitative Pharmacology and Pharmacometrics, Cecilia Fosser, showcased innovative work on creating model based predictions of pharmacodynamic responses in ulcerative colitis patients. Fosser presented a poster Model based predictions of the PTG-100 pharmacodynamic responses in ulcerative colitis patients created in collaboration with colleagues from Protagonist Therapeutics.
In this blog we share the abstract and link to access an electronic copy of the poster .
Abstract
Title: Model based predictions of the PTG-100 pharmacodynamic responses in ulcerative colitis patients
Author: C. Fosser (1), L. Mattheakis (2), R. Saralaya (1), K. Horsch (1), N. Rao (2), L. Bai (2), L. Zhao (2), T. Annamalai (2), D. Liu (2)
Institution: (1) Cytel Inc., Cambridge, United States, (2) Protagonist Therapeutics, Milpitas, United States
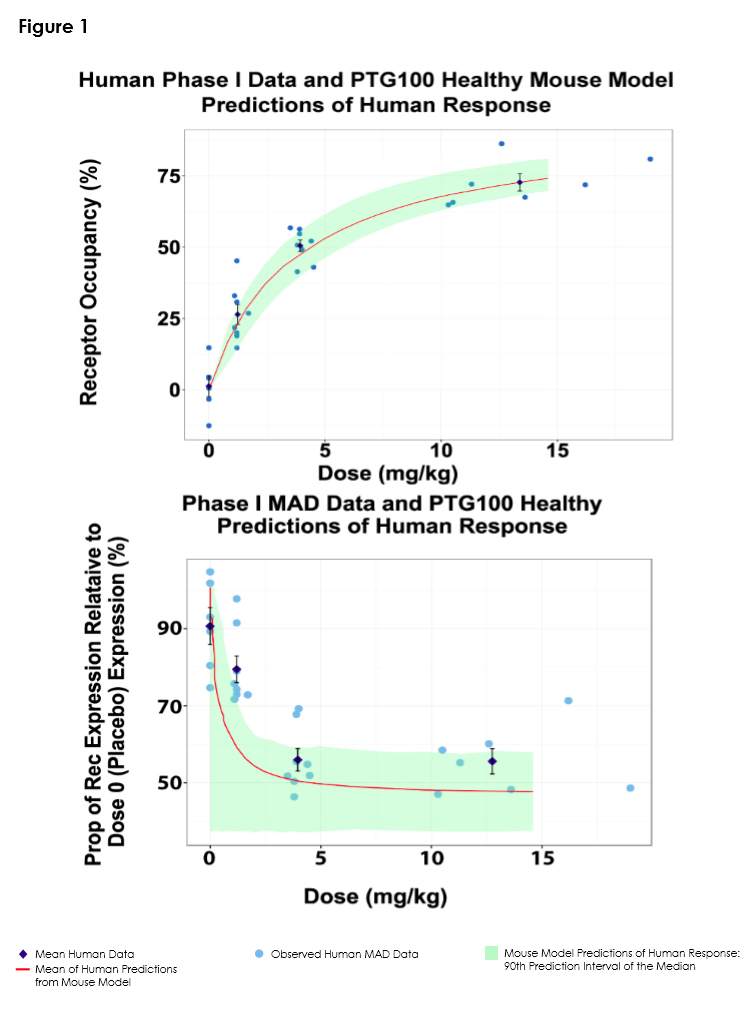
Objectives: To develop exposure (dose)-response (DR) models of healthy and colitis mice, and Phase 1 healthy volunteers (HV) for α4β7 receptor occupancy (RO) and receptor expression (RE) in the peripheral blood, and then to leverage all three models together to predict colitis patient response to PTG-100. PTG-100 is an oral peptide antagonist that binds specifically to α4β7 integrin on CD4+ memory T cells and alters their trafficking to gut tissues. It is currently in clinical development for ulcerative colitis. 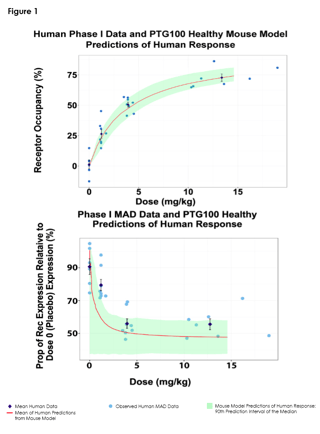
Methods: Pharmacokinetic (PK) and Pharmacodynamic (PD) data were obtained from pre-clinical healthy (N=30) and diseased (N=55) mice studies and from a Phase 1, double-blind placebo-controlled single-ascending (N=30) and multiple-ascending (N=30) dose study in HV. Semi-mechanistic, nonlinear, mixed effects models were used to characterize the PD response observed in healthy and colitis mouse studies, and in the Phase 1 HV study and implemented in Phoenix NLME software [1]. Colitis mouse RO response modeling consisted of the introduction of a non-symmetry parameter, ψ, in the Emax modeling structure [2]. Key parameters were linked between healthy and colitis mouse models, and these links were used to predict patient PD responses from the HV PD responses.
Results: RO and RE DR relationships were well characterized with a sigmoidal emax model, and a sigmoidal inhibition model, respectively, in healthy and colitis mice, and in HVs. The colitis mouse model was structurally connected with the healthy mouse model by estimating colitis mouse multipliers applied to typical values of key healthy mouse parameter estimates, and this was used to extend the HV models to predict colitis patient responses.
Conclusions: The modeling was able to characterize and leverage the differences and similarities between healthy and colitis mouse DR relationships to extrapolate the HV DR relationship enabling predictions of the colitis patient DR relationship.
References:
[1] Certara’s Pharsight Phoenix NLME 7.0, Princeton, New Jersey
[2] F. J. Richards, A Flexible Growth Function for Empirical Use, Journ. of Experimental Botany, Vol. 10, No 39, pp. 290-300, June 1959.


