Editor's note( this blog was refreshed in April 2018)
As CDISC compliant submissions become increasingly expected, biopharmaceutical companies are considering how to approach the issue of data standards governance. Standards governance is a lynchpin in the management of CDISC compliance and is important for promoting standards awareness within organizations. It’s also an acknowledged hot topic in the industry.
It has traditionally been common practice for biopharma companies to outsource their CDISC conversion of legacy data for the purpose of publications and submissions to expert CROs. While large biopharma organizations may have dedicated in-house teams deployed to the management of standards governance, the dynamic nature of CDISC requirements means companies can struggle to find the resources to keep up to date and provide the best interpretation of the documentation. Outsourcing can be an option to ensure dedicated staff are available to manage and monitor these aspects and ensure companies remain submission ready.
A dedicated standards governance committee will typically be multidisciplinary across the sponsor’s clinical research team and may also include input from the CRO.
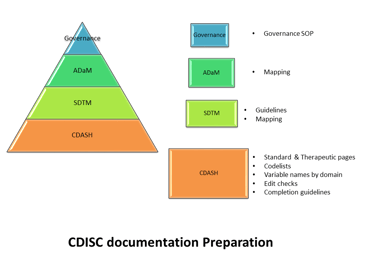
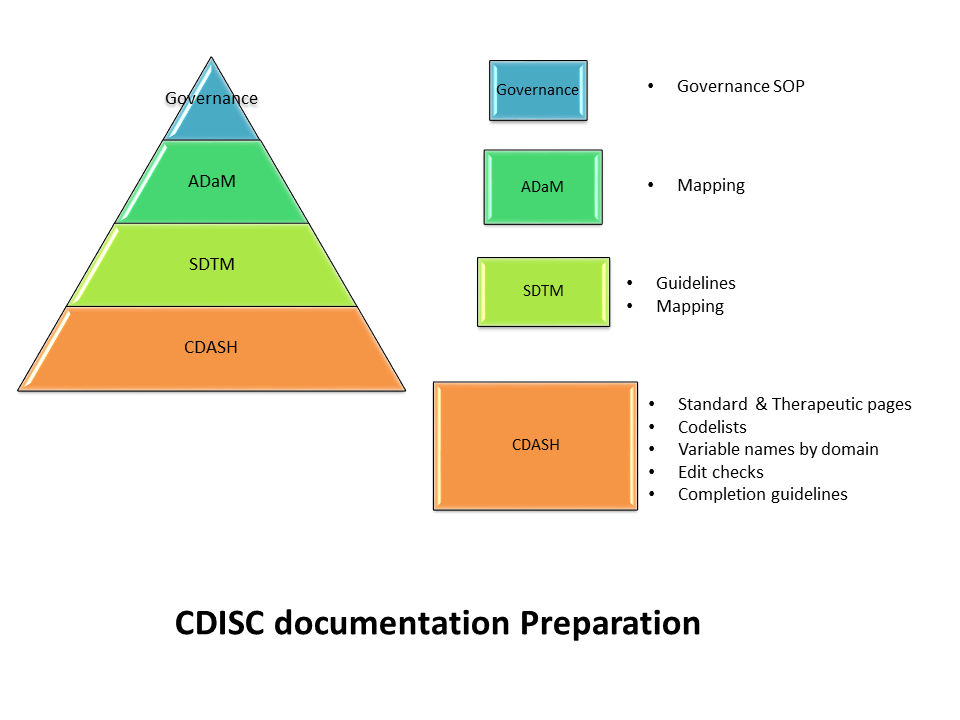
The committee will oversee an organization’s approach to:
- Standard CRF pages/ CDASH
- SDTM structures
- ADaM structures
as well as decisions around the following aspects:
Application of data standards libraries( in particular when outsourcing to CROs).
One of the issues which is important to address as part of standards governance is how the standards should be deployed by CROs, and ensuring compliance with those standards. An effective model is for a CRO to be contracted to be responsible for standards governance, and effectively oversee the adherence to those standards by the CROs responsible for the creation of datasets and outputs for specific studies. This can provide a solution for a biopharma company without sufficient resources for an in-house governance team, while still maintaining the integrity and compliance to their standards.
How deviations from the existing data standards will be managed, and how to track which version of the data standards is used for each study.
Where any deviation occurs from the standards, this would be referred back to the governance committee and a decision made on how to address the deviation. This can also be taken as a learning point moving forward.
How and when the data standards will be updated, versioned, and changes controlled.
CDISC amendments related to data collection through to analysis, and across therapeutic areas are released on an ongoing basis. Monitoring of these ongoing amendments, assessing their relevance to the organization, interpreting and implementing is a resource intensive process. The governance committee would be responsible for reviewing and deciding whether a specific amendment is something which they would want to incorporate. The amendments can be open to interpretation, and therefore having the right expertise involved, capable of understanding the amendments significance to a particular organization is crucial. Frequently, CROs will have broad CDISC experience across therapy areas due to the variety of their work and are in a good position to offer expert help.
To find out more how our CDISC experts can help click here: