
Perspectives on Enquiry & Evidence
Cytel's blog featuring the latest industry insights.

July 27, 2022
Written by Jing Ping Yeo and Charles Warne Adaptive designs are studies that “include a prospectively planned...
Read article

June 28, 2022
Career Perspectives: Reflecting at 10 Years in Cytel FSP
Founded in 1987 by Cyrus Mehta and Nitin Patel, research scientists at Harvard University and MIT respectively and...
Read article

March 18, 2022
The Fundamentals of Real World Evidence in Oncology Drug Development
Real world evidence (RWE) provides a large and growing source of insights into drug uptake and safety. It is...
Read article

March 11, 2022
WINTER WEEKEND READ: Increased Adoption of Innovative Designs
Every year, sponsors hesitating to use a complex innovative clinical trial design routinely miss opportunities to...
Read article

February 18, 2022
WINTER WEEKEND READ: Role of Data Strategy in Clinical Development Success
Data is the most crucial asset of any clinical trial and hence, sponsors cannot jeopardize collecting clean...
Read article

February 11, 2022
WINTER WEEKEND READ: Tops Tips and Tricks from the Good Data Doctor
Adopting data standards such as CDISC in the early phase of clinical drug development contributes to the consolidation...
Read article

February 4, 2022
WINTER WEEKEND READ: Model-Based Enrollment Forecasting
The ability to conduct data-driven and quantitatively rigorous feasibility studies, is often key to successful trial...
Read article

January 21, 2022
How to Use and Interpret the Results of a Platform Trial
For our first Winter Weekend Read, Cytel presents How to Use and Interpret the Results of a Platform Trial, a JAMA...
Read article

December 23, 2021
Year-End Roundup: Your Favorite Blog Posts of 2021
Cytel blogs bring you debate and discussion of the newest trends in statistics and quantitative strategy. In 2021, our...
Read article

December 14, 2021
Is Promising Zone Design Optimal?
In traditional clinical trial design, the sample size is often determined to detect the target treatment effect with...
Read article

April 29, 2021
Advances in HEOR: An Interview with Anna Forsythe
After twenty years in pharma, Anna Forsythe was frustrated by traditional vendors who used outdated methods to prepare...
Read article
April 26, 2021
Why you should not miss 2021 Virtual CDISC EU Interchange?
As we all continue to take necessary precautions against the spread of COVID-19 virus, this year again the CDISC EU...
Read article

March 31, 2021
Wearables and Decentralization
As decentralized clinical trials become more attractive in an era of COVID-19, the role of wearables in clinical...
Read article

March 30, 2021
The Integration Dilemma
As of today, our Industry has not defined any approach, nor does an official regulatory agency...
Read article

March 26, 2021
Using External Evidence for Decision-Making for Medical Devices
Former Commissioner of the FDA, Dr. Scott Gottlieb, in several public presentations, would bemoan missed chances to...
Read article

March 25, 2021
US Regulatory Aspects of Wearable Medical Devices
Wearables have experienced increasing applicability within medical device trials, yet the regulations for the use of...
Read article

March 19, 2021
5 Steps to Regulatory Success with Wearables Designs
The use of wearable and digital technology requires considerations for both drugs and devices regulations, and...
Read article

March 18, 2021
Computing & Statistics: Are You Ready for the Industry Transformation?
Over the past ten years High-Performance Computing (HPC) has transformed medical research through advances in genomics,...
Read article

March 12, 2021
Wearables: Translating raw data to actionable information
With wearables likely to become a regular part of clinical trial design, statisticians could benefit by familiarizing...
Read article

March 11, 2021
Data and analysis in Modern Oncology Clinical Development
In the recent years, Oncology trials are seeing a technological shift that is expected to make them faster and more...
Read article

March 9, 2021
Seeing Uncertainty: New Frontiers of Statistical Communication
When statistical sciences were in their infancy, the communicative benefits of statistics were widely touted. Thousands...
Read article

March 2, 2021
Hybrid Bayesian and Frequentist Clinical Trial Designs
Most people know that clinical drug discovery is usually conducted using either Frequentist or Bayesian methods. These...
Read article
February 26, 2021
Empowering Statisticians to Create Complex Bayesian Clinical Study Designs
In the world of clinical trials, the pace of innovation is accelerating, and approaches such as Bayesian methods are...
Read article

February 25, 2021
Use of Wearables in Confirmatory Clinical Trials
The convergence of several distinct trends has made wearables an increasingly attractive option for use in confirmatory...
Read article

February 24, 2021
Avoiding Lost-in-Translation with Submission Terminology
In a previous post, I discussed the importance of proper use of CDISC Controlled Terminology (CDISC CT) in SDTM....
Read article

February 19, 2021
Selecting Your Next Clinical Trial Design Using Quantitative Methods
C-Suite and R&D Decision-Makers are always striving to make evidence-driven decisions. Yet the rules by which evidence...
Read article
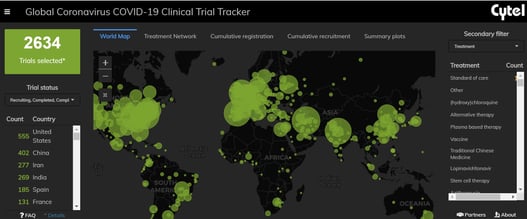
February 17, 2021
An Interview with Louis Dron on the Benefits and Future of Cytel’s Trial Tracker
The COVID-19 Pandemic prompted the rapid surge in the generation of clinical data that has been scattered across...
Read article

February 12, 2021
Leveraging Synthetic and External Control Arms Using Bayesian Methods
In recent times, Single arm trials are being increasingly used to assess new treatment interventions. They establish...
Read article

February 11, 2021
The biostats and clinical overview of a growing clinical strategy
The past two years have witnessed a heightened interest in the use of wearables in clinical development. The unexpected...
Read article

February 9, 2021
New Meta-Analysis in JAMA Uses Novel Quantitative Techniques to Demonstrate Baseline Characteristics Informing Response to Common Therapy for Kidney Cancer
Recent years have witnessed improving survival outcomes for those struggling with a range of common kidney cancers....
Read article

February 4, 2021
Simulation Based Clinical Trial Optimization
The past decade has witnessed the rise of simulations-based clinical trial optimization in a manner unimaginable to...
Read article

February 2, 2021
Bayesian Methods for Master Protocols
As the use of master protocols becomes more prevalent in drug development, Bayesian methods are extensively used to...
Read article
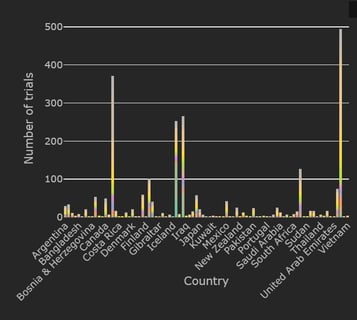
February 1, 2021
February 2021: Updates from the CYTEL COVID-19 Trial Tracker
Cytel’s COVID-19 Trial Tracker continues to provide real time updates to the status of COVID-19 clinical trials...
Read article
January 29, 2021
Computation and Clinical Trial Design: New Directions
Historically, advances in the statistical design of clinical trials have accompanied progress within the science and...
Read article

January 28, 2021
A little walk in the CDISC Library, hand in hand with SAS
The Christmas break presented an opportunity to make my first concrete steps into the CDISC Library. Overall, it was a...
Read article

January 22, 2021
The Role of Real World Evidence after COVID19
COVID-19 has transformed the pharmaceutical industry in a manner that few could have predicted only a year ago. One of...
Read article

January 20, 2021
Quantifying Tradeoffs in Clinical Development
One of the most difficult challenges facing Research and Development teams involves determining how to make tradeoffs...
Read article

January 19, 2021
Cytel COVID Panel: Long-term Changes to Clinical Trials Due to the Pandemic
As we enter 2021 with new COVID-19 vaccines and greater optimism about the pipeline of drugs and devices positioned for...
Read article

January 13, 2021
How to Create a High-Quality Globally Distributed Biometrics Team?
Effective use of the right outsourcing solution can enable sponsors to respond to market needs and change course where...
Read article
January 12, 2021
5 Questions to Help You Modernize Clinical Development
The rapid pace of technology has opened up numerous avenues for advanced innovative clinical trial design, but how can...
Read article
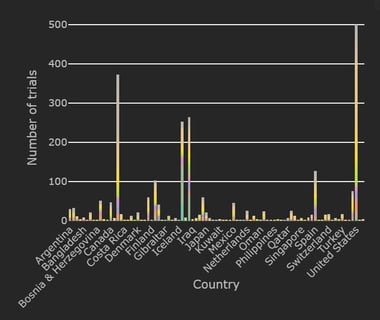
January 11, 2021
COVID-19 Trial Tracker Updates (January 11)
In April 2020, Cytel launched an open-access global COVID-19 Clinical Trial Tracker to help facilitate greater...
Read article

December 22, 2020
Year-end Roundup: Cytel’s Contributions Towards Health & Education in 2020
At Cytel, we have been diligently working to become an organization deeply committed to uplifting and enriching...
Read article

December 21, 2020
Year-End Roundup: Your Favorite Blog Posts of 2020
2020 has been an unusually difficult year as the global pandemic impacted all of our lives. This year, the Cytel blog...
Read article

December 18, 2020
Submitting Software Programs to the Regulatory Agencies
Can I submit software programs other than SAS? What software programs should I submit? Are sponsors required to submit...
Read article

December 17, 2020
2020 Recap by Yannis Jemiai, Chief Scientific Officer, Cytel
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

December 16, 2020
2020 Recap by Pantelis Vlachos, Principal/Strategic Consultant, Cytel
As we prepare to close the door on 2020, we asked Pantelis Vlachos, Principal/Strategic Consultant for Cytel, to share...
Read article

December 15, 2020
Satisficing, Optimizing and Globally Optimizing Trial Designs
When designing clinical trials, biostatisticians and clinical development teams are often faced with a conundrum. Given...
Read article

December 10, 2020
Career Perspectives: Interview with Sachin Sobale
Sachin Sobale began his career with Cytel as a young statistician. He has been associated with the company for more...
Read article

December 9, 2020
7 Key Features of Strategic Clinical Trial Design
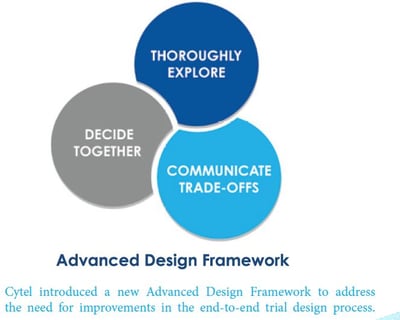
As a part of Cytel’s Advanced Design Framework, a new Framework for the statistical design of clinical trials, Cytel...
Read article
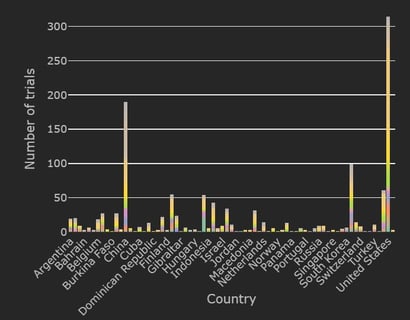
December 8, 2020
COVID-19 Trial Tracker Updates (December 8)
The Cytel COVID-19 Trial Tracker brings you an up to the minute, real time dashboard about COVID-19 trials around the...
Read article

December 3, 2020
New Whitepaper: Reimagining Clinical-Trials
Increasing Clinical Development Productivity Using Statistics and Cloud-Computing The need for Re-imagining Clinical...
Read article

December 2, 2020
Program and Portfolio Optimization: A New Paradigm
Significant advances have been made to enhance the efficiency of clinical trial designs. However, the traditional...
Read article
December 1, 2020
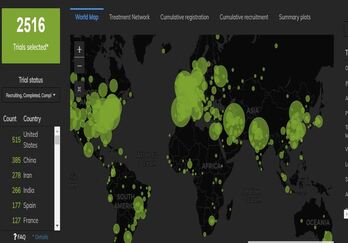
Mapping the Landscape of COVID-19 Clinical Trials in the US
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread. As...
Read article

November 24, 2020
Cytel and Ingress Health at Virtual ISPOR Europe 2020
Virtual ISPOR 2020, held November 16 to 19, presented new opportunities for scientific interaction amongst HEOR...
Read article

November 23, 2020
Data Standards and Submission Highlights from PHUSE EU CONNECT 2020
The Virtual PHUSE-EU CONNECT Conference was held from November 8 to 13 and the event was a great success, despite all...
Read article

November 19, 2020
10 Key Qualifications for Independent Statisticians Reporting to the DMC
Data Monitoring Committees (DMCs) are groups of independent experts who periodically receive (by-arm) reports created...
Read article

November 18, 2020
We can design over 100,000 clinical trials in less than an hour
The current state of the clinical trials industry faces a challenge that was only hypothetical three or four years ago....
Read article
November 17, 2020
Role of Prediction and Causal Inference in Clinical Research
As a part of Cytel’s "New Horizons Webinar Series", Alind Gupta, Senior Data Scientist, presents case studies from his...
Read article

November 16, 2020
Bayesian Methods for Multiple Cohort Expansion (MuCE) designs
MUCE is a Bayesian solution for cohort expansion trials where multiple dose(s) and multiple indication(s) are tested in...
Read article

November 12, 2020
Join Cytel and Ingress Health at Virtual ISPOR Europe 2020
Cytel and Ingress Health (now a Cytel company) will be contributing to a range of events at Virtual ISPOR EUROPE 2020,...
Read article

November 11, 2020
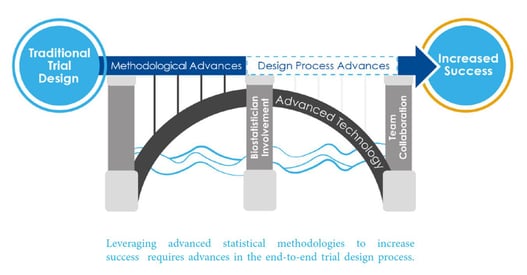
Interview with Yannis Jemiai: Advanced Design Framework
The widespread use of cloud-computing has altered the clinical trial design process. Whereas three or four years ago,...
Read article
November 10, 2020
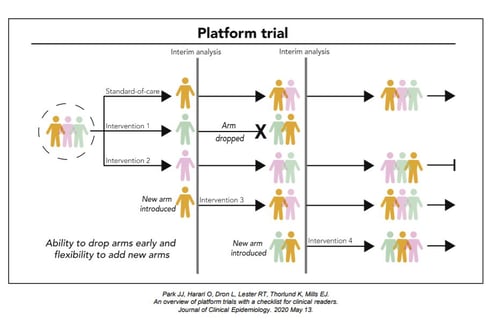
Key Design Considerations for Platform Trials
Platform trials are a new type of clinical trials where multiple interventions can be evaluated simultaneously against...
Read article

November 5, 2020
Role of RWA in Transforming Oncological Research
In oncology, many manufacturers go into niche indications, where there are very specific tumors, and then they opt for...
Read article

November 4, 2020
Cytel Introduces Advanced Design Framework: Part 3 - Communication Techniques to Ensure Alignment on Data-Driven Clinical Trial Designs
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought leaders that draws on...
Read article

November 3, 2020
Staffing Needs for RWE Delivery
When an expert statistician is paired with an experienced set of data managers, opportunities to capitalize on...
Read article

November 2, 2020
Value of Detailed Clinical Trial Simulations for Rare Diseases
Measuring treatment effect during a clinical trial is often the source of much debate, particularly during rare disease...
Read article

October 29, 2020
Advanced Design Framework: Part 2 - A Quantitative Evaluation Approach
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought leaders that draws on...
Read article

October 28, 2020
When your ADaM package is not traceable back to SDTM
About three years ago, Cytel was helping a sponsor on a project where I had to conduct surveillance of some CRO...
Read article

October 27, 2020
Bayesian Dose-Finding Designs – An Overview
Cytel recently conducted a webinar on Bayesian Dose-finding Designs for Modern Drug Development, presented by Dr. Yuan...
Read article

October 26, 2020
Need for Technology Solutions to Support Computationally
Pharmaceutical and biotech companies are under pressure to deliver more and deliver faster with fewer resources. The...
Read article

October 23, 2020
Yuan Ji on U-Design: An All New Efficacy and Toxicity Dose-Finding Module
Cytel’s New Horizons Webinar Series introduces you to the latest innovations in statistical trial design. This webinar...
Read article

October 22, 2020
An Interview with Bart Heeg (Part 2): New Trends in HEOR
In this two-part blog series, we interview Bart Heeg, Vice President HEOR and Founder at Ingress Health (A Cytel...
Read article

October 21, 2020
Advanced Design Framework: Part 1 - Exploration of Design Space
Cytel has recently revealed its Advanced Design Framework, a method developed by Cytel’s thought-leaders after a decade...
Read article

October 20, 2020
Interview with Thomas Wilke: Health Economics/World Evidence Studies
In this interview with Thomas Wilke, Principal Scientist at Ingress-Health (a Cytel company), we talk to him about his...
Read article

October 19, 2020
The Uniqueness of COVID-19 Data Challenges; The COVID-19 trial tracker
COVID-19 has created extreme uncertainties -- a dearth of historical information combined with the need for safety,...
Read article

October 15, 2020
An Advanced Design Framework for Clinical Development in the Era of Cloud-Computing
For over a decade, advanced trial design techniques have promised efficient trials with accelerated timelines,...
Read article

October 14, 2020
The Increasing Importance of Health Economics
A credible evidence base is needed to support and document the economic value of new technologies and therapeutic...
Read article
October 13, 2020
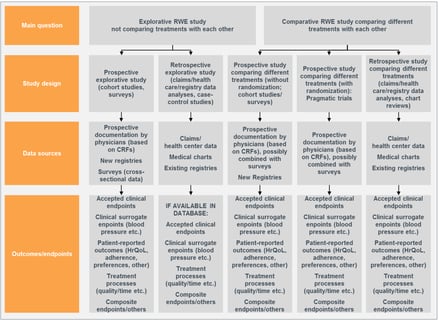
Introducing Observational Studies – Three Trends for Statisticians
The combination of greater access to electronic health records, bigger electronic claims datasets, and the need for...
Read article

October 12, 2020
Bayesian Statistics and FDA Regulatory Acceptability
Cytel and Novartis are together hosting a complimentary Bayesian Virtual Symposium and an Interactive 7-part workshop....
Read article

October 9, 2020
Cyrus Mehta on Increasing the Power of Platform Trials
Even before the era of COVID-19, significant attention was channeled to the overwhelming potential of adaptive MAMS...
Read article

October 7, 2020
RWE Needs for Natural History Studies
With the rise in digital technologies, there has been an explosion in the volume and type of data sources. We can...
Read article
October 1, 2020
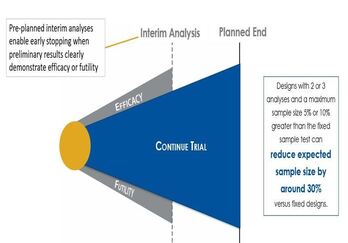
Improve Trial Design with Sequential Design and Sample Size
Methods involving Group Sequential Designs are one of the earliest deviations from a traditional two-arm clinical trial...
Read article

September 30, 2020
A Virtual Event Brought to you by Cytel and Novartis on Innovations
Today, there is a need for advanced quantitative techniques to combine all available information for better decision...
Read article

September 29, 2020
The New Horizons Series: Adaptive Multi-arm Multi-stage Clinical Trials
Innovation in trial designs are offering new routes forward for organizations of any size. They are now also aligned...
Read article

September 28, 2020
Advantages of platform designs for investigating COVID-19 therapies
Cytel has recently designed and implemented the TOGETHER Trials, funded by the Bill & Melinda Gates Foundation to...
Read article

September 25, 2020
Use of External Controls in Clinical Development – Download Audiobook
Regulators in both the United States and Europe have responded positively to the use of synthetic control arms (SCA)s...
Read article

September 22, 2020
LIBERTY claim analysis manuscript by Thomas Wilke and Sabrina Mueller
Research Scientists, Thomas Wilke and Sabrina Mueller recently published a manuscript on “Diabetes-Related...
Read article

September 21, 2020
Novel Adaptive Platform Trial for COVID-19 Therapies
Cytel has designed and implemented a novel adaptive platform trial for early stage COVID-19. The severity of the...
Read article

September 17, 2020
East Alloy: Accelerating the pace of innovation
Keeping up with the rapid pace of clinical development means that we need to adopt the innovative or computationally...
Read article

September 16, 2020
New Audiobook on Synthetic Control Arms
Synthetic control arms (SCA) are virtual trial arms that use historical claims data and observational data to simulate...
Read article

September 15, 2020
Accurately analyze small, skewed or sparse data with StatXact
In clinical trials with small or sparse data, statistical methods meant for large sample sizes may not be helpful to...
Read article

September 14, 2020
From Before to After: Preparing and Concluding your FDA Data Submission
“A good start is half the battle” (the Before) when submitting data to the FDA and there are a couple of cherries to...
Read article

September 10, 2020
Does your Trial need a Synthetic Control Arm?
Single arm trials are emerging as an accepted way of assessing a new treatment intervention. They establish clinical...
Read article

September 9, 2020
Importance of Designing Clinical Trials from a Program Perspective
Cytel’s co-founder, Nitin Patel, conducted a webinar on designing clinical trials from a program-level perspective. His...
Read article

September 4, 2020
Cytel Co-Founder Cyrus Mehta Presents at the Heart Failure Collaboratory, a Public-Private Partnership with FDA
On Friday September 11, Cyrus Mehta, co-founder of Cytel, will be delivering a talk to the Heart Failure Collaboratory,...
Read article
September 1, 2020
FSP Navigator – Building the Team that Brings Success
In this blog, Cytel's SVP Corey Dunham’s talks about our Functional Services teams and the qualities we seek in new...
Read article

August 31, 2020
Adopt innovative and computationally intensive designs with East Alloy
Pantelis Vlachos, Principal, Strategic Consultant at Cytel, conducted a webinar to introduce the capabilities of East...
Read article

August 26, 2020
Career Perspectives: Interview with Yannis Jemiai, Chief Scientific Officer
As Chief Scientific Officer, Dr. Yannis Jemiai plays a pivotal role in maintaining Cytel’s well-established reputation...
Read article

August 25, 2020
Nitin Patel on Designing Clinical Trials from a Program Perspective
It is important to take a strategic approach to clinical development in order to minimize the potential for Phase 3...
Read article

August 21, 2020
Bayesian Borrowing and Real World Data: The Fundamentals
As uses of real world data become more familiar for trial design and regulatory submission, sponsors might become more...
Read article

August 19, 2020
Webinar on Adaptive Designs for Dose Finding: Part 2
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article

August 18, 2020
Career Perspectives: Interview with Mrudula Joshi, Associate Director, Statistical Programming Services
Mrudula Joshi joined Cytel in July 2005 as a young SAS programmer. Last month, she celebrated her 15th year work...
Read article

August 13, 2020
Webinar: Adaptive Designs for Dose Finding
Bjoern Bornkamp, Statistical Methodologist at Novartis and Jose Pinheiro, Senior Director, Johnson & Johnson provided...
Read article

August 12, 2020
Virtual Careers Open Day at Cytel
Cytel’s Biostatistics and Statistical Programming team provides integrated solutions, by blending the expertise of...
Read article
August 11, 2020
Optimizing Information in Trial Design and Implementation
While there is increasing optimism about the discovery of a COVID-19 vaccine, one of the less talked about aspects of...
Read article

August 7, 2020
Creating a Synthetic Control from Your Natural History Study
Recently a biotech approached Cytel for support with a Phase 2 Study in oncology. Regulators had requested a natural...
Read article

August 5, 2020
Design and Data Considerations from Cardiovascular Pilot Investigation
Cytel is conducting two pilot projects on head-to-head comparisons using real world data. These projects in oncology...
Read article

August 4, 2020
Estimands and their Implications on Clinical Studies
Last year, Paul Terrill, Associate Principal of Strategic Consulting at Cytel, presented an engaging webinar on the...
Read article

July 30, 2020
Cytel Scientists Call for a “Statistician-First Workflow” to Optimize Drug Development
A new peer-reviewed article co-authored by several Cytel scientists re-examines the way in which adaptive trials are...
Read article

July 28, 2020
Therapeutic Area User Guidance – The hidden Gems
CDISC standards have been around for a while with the first SDTM Standard version released in 2004. However, it was...
Read article

July 27, 2020
Introduction to Population Enrichment by Dr. Thomas Burnett
Cytel is conducting a webinar series on complex innovative trial designs. Dr. Thomas Burnett, Senior Research Associate...
Read article

July 23, 2020
Why Consider a Synthetic or External Control Arm?
Just as there are numerous adaptations that fall within the umbrella of adaptive designs, there are several different...
Read article

July 22, 2020
Access Sustainable, Verified Innovation with East Alloy
Cytel brings to you a new blog series on technology and Bayesian decision-making by Pantelis Vlachos,...
Read article

July 21, 2020
Head to Head Comparisons Using Real World Data
Cytel is conducting a webinar series that focuses on target trial emulation and causal inference approaches using real...
Read article

July 15, 2020
Three Reasons Why Oncology Trials Need Clear Estimands
Unlike many therapeutic areas, oncology benefits from having standardized endpoints like overall survival and...
Read article

July 14, 2020
The FSP Engagement Landscape: Reducing Sponsor Oversight Burden
Cytel SVP Corey Dunham’s inaugural post on leading the industry’s largest Biometrics CRO considers the FSP Engagement...
Read article

July 13, 2020
Interview with Dr. Thomas Burnett on Adaptive Enrichment
Cytel is hosting a complimentary webinar series that introduces biostatisticians and other members of the development...
Read article

July 9, 2020
Synthetic control arms demystified: A Cytel Ebook
There has been an increased use of synthetic control arms for regulatory submissions in recent years, with three rare...
Read article

July 7, 2020
Overcoming Clinical Development Challenges in Oncology with Innovative
Having its roots in the seminar rooms of the Dana Farber Cancer Institute, Cytel has a long record of establishing new...
Read article

June 29, 2020
The Good Data Submission Doctor: CDISC for COVID-19
From the time the COVID-19 outbreak was declared a pandemic, the number of studies conducted around the world to either...
Read article

June 27, 2020
New Whitepaper: Bayesian Methodologies for COVID-19 Drugs
Expert statisticians at Cytel have spent the past three and a half months designing and deploying dozens of trials for...
Read article

June 24, 2020
Webinar - Practical Model-based Approaches for Phase I Oncology Trials
Last week, Cytel conducted its third webinar in the new introductory webinar series on Complex Innovative Trial...
Read article
June 23, 2020
Design and Data Source Considerations from Pilot Investigations in CVD
Supposing two treatments, A and B, need to be compared that have not been compared through a clinical trial. In the...
Read article

June 22, 2020
Webinar: Synthetic and External Controls in Clinical Trials
Cytel scientists recently published a new eBook on synthetic control arms and a new scientific primer for the more...
Read article

June 18, 2020
Why You Should Construct Primary Endpoints Using Bayesian Methods
One of the revelations of the COVID-19 pandemic is that the flexibility and potential of Bayesian designs goes far...
Read article

June 18, 2020
Optimizing Patient Recruitment: Download Whitepaper
A number of trials recently disrupted by the COVID-19 pandemic are now in the process of re-assessing recruitment...
Read article

June 15, 2020
Significance of Bayesian Model-Based Approaches in Oncology Trials: An Interview with Dr. Satrajit Roychoudhury
Cytel conducted a webinar with Dr. Satrajit Roychoudhury, Senior Director, Statistical Research and Data Science...
Read article
June 11, 2020
Adaptive Bayesian Methods: The Secret Weapon in COVID-19 Vaccine Development
A recent Cytel panel led by Vice President of Strategic Consulting Natalia Muhlemann evaluated the role that Bayesian...
Read article
June 10, 2020
Group Sequential Designs and Sample Size Re-estimation
Cytel is conducting a webinar series that introduces biostatisticians to some of the more commonly used complex...
Read article
June 9, 2020
Melinda Gates, on COVID-19 and Drug Development in Emerging Economies
Trevor Mundel leads the Bill & Melinda Gates Foundation’s efforts to develop high-impact interventions against the...
Read article

June 8, 2020
New Primer and Ebook on Synthetic Control Arms
Cytel has recently published a new ebook on synthetic control arms, and a new scientific primer as well.
Read article
June 1, 2020
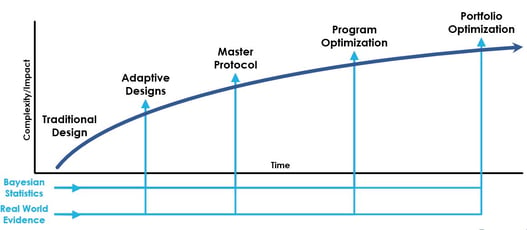
Webinar Replay: Innovative Drug Development at a Glance
In a recent interview with Cytel, Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design...
Read article

May 28, 2020
Implications for the Future of Drug Development in Emerging Economies
On May 7, Cytel and Certara conducted a virtual panel discussion on new opportunities and implications for the future...
Read article

May 27, 2020
Group Sequential Designs and Sample Size Re-estimation
In this blog, we speak with Christopher Jennison, Professor of Statistics at the University of Bath, UK. Professor...
Read article

May 18, 2020
Interview with Zoran Antonijevic on Adaptive Design Methods
In this blog, we speak with Zoran Antonijevic, longstanding chair and leader of the DIA Adaptive Design Scientific...
Read article

May 12, 2020
Oncology Trial Design & Development Webinar Series
In our previous blog, “Remote Working Arrangement – How to get it right?”, we talked about how the need for social...
Read article

May 7, 2020
COVID-19: Trials, Designs and Tools for Promising Results - A Virtual Panel Discussion
An extraordinary amount of global research is underway as the COVID-19 pandemic continues to evolve and spread...
Read article

May 5, 2020
Webinar: A Clinician’s Perspective on Cancer Drugs Development
Cytel's team of oncology trial design and advanced analytics experts are hosting a series of complimentary webinars...
Read article
April 29, 2020
Webinar: Transparent Machine Learning in Oncology
In our previous blog, we spoke with Alind Gupta, who works as a Machine Learning Researcher at Cytel in Canada. The...
Read article

April 27, 2020
Highlights from the 2020 Virtual CDISC EU Interchange - Part 2
In the first part of this two-parts blog, I speak about how the European CDISC Committee (E3C) together with CDISC...
Read article

April 24, 2020
Highlights from the 2020 Virtual CDISC EU Interchange by Angelo Tinazzi
In early March, when countries around the world started implementing lockdowns, the European CDISC Committee (E3C)...
Read article

April 23, 2020
A Clinician’s Perspective on Cancer Drugs Development
Cytel is hosting a webinar, “A Clinician’s Perspective on Cancer Drugs Development”, on April 28, 2020. Our speaker,...
Read article
April 23, 2020
Weekly Insights from the COVID-19 Trial Tracker
Every Week Cytel Brings You Further Insights from the COVID-19 Trial Tracker From April 8 through April 17, the number...
Read article

April 20, 2020
Interview with Alind Gupta: Transparent Machine Learning in Oncology
Cytel is hosting a webinar on Transparent Machine Learning in Oncology, on April 21, 2020. Our speaker, Alind Gupta,...
Read article

April 7, 2020
Career Perspectives: Interview with Marc Lefebvre-Gouy, Statistical Programmer
Cytel has industry-leading experts in Statistical Programming with years of SAS® Programming expertise and in-depth...
Read article

April 2, 2020
Remote Working Arrangement – How to get it right?
On March 16, the World Health Organization (WHO) Director-General, Dr. Tedros Adhanom Ghebreyesus, in his media...
Read article
March 31, 2020
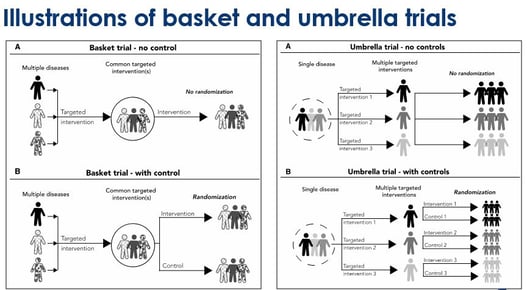
Key Design Thoughts for Basket Trials and Umbrella Trials by Jay Park
Since 1953, when the discovery of the structure of DNA was made, we have seen great advancements in genomics....
Read article
March 26, 2020
Cytel's Response: EMA Points to consider on implications of COVID-19
Further regulatory guidance has been released concerning the implications of the Coronavirus disease (COVID-19) on...
Read article

March 23, 2020
Cytel's Response: EMA Guidance on the Management of Clinical Trials During the COVID-19 (Coronavirus) Pandemic
On March 20th the European Commission, the European Medicines Agency (EMA) and the Heads of Medicines Agency (HMA)...
Read article

March 19, 2020
Cytel's Response: FDA Guidance on Conduct of Clinical Trials during the COVID-19 Pandemic
The FDA issued a guidance yesterday on how the COVID-19 Pandemic may affect the conduct of clinical trials. Below are...
Read article

March 12, 2020
Interview with Jay Park: The present and future of Master Protocols
In September 2018, the FDA provided a draft guidance on master protocols reflecting an increased interest in these...
Read article

March 5, 2020
Managing risk in clinical development: Is your data strategy fail-safe?
Generating high-quality clinical data is a vital but challenging task in modern drug development. Unfortunately, in the...
Read article

February 20, 2020
Unlock the power of your clinical data with these five top tips
It is widely acknowledged among drug developers that one of their most important assets is the data generated during...
Read article

February 6, 2020
Is your data strategy set up to tackle key challenges in early clinical development?
In clinical development, a high-quality evidence package is a prerequisite for a new therapy to gain approval from...
Read article
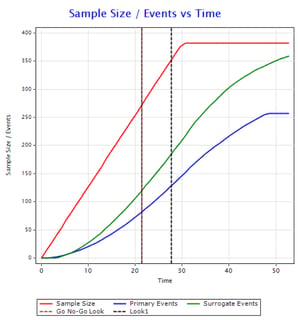
January 30, 2020
Designing Event-based Studies: Interview with Pantelis Vlachos
The Cytel Trial Design Innovations (CTDI) Webinar Series recently hosted a webinar on designing event-based studies....
Read article

January 22, 2020
What could you accomplish with a fresh approach to your clinical data strategy?
In the quest for clinical success, we all strive for evidence packages of the highest quality. If the clinical data is...
Read article

January 16, 2020
Adaptive Population Enrichment in a Phase III Oncology Trial
January’s Cytel Trial Design Innovations (CTDI) Webinar Series will feature Biostatistician and pioneering Bayesian...
Read article

January 8, 2020
How to optimize your data strategy to drive success in clinical development
In clinical development, data is the vital ‘foundation’ that supports your programs. To successfully bring a promising...
Read article

December 18, 2019
Year-End Roundup: Your Favorite Blog Posts of 2019
With only two weeks left for this fabulous year to end, we would like to thank all our blog subscribers and new readers...
Read article

December 16, 2019
Career Perspectives: Interview with Ronald Dumpit, Data Coordinator
Cytel Inc. and Axio Research joined forces in June 2019, expanding our ability to solve the most complex analytical...
Read article

December 10, 2019
Impact of AI on Clinical Development
In association with Statisticians in the Pharmaceutical Industry (PSI) , UCB and Cytel hosted a symposium on September...
Read article

December 5, 2019
Biotechs and Medtechs, don’t forget your market access strategy (part 4 of 4): How to optimize your market access planning approach
Author: Michael S. Paas, Market Access & Commercialization Expert, Executive at AbbVie and Guest Author at Cytel In...
Read article

December 2, 2019
Biotechs and Medtechs, don’t forget your market access strategy (part 3 of 4): Harnessing the value of market access planning
Author: Michael S. Paas, Market Access & Commercialization Expert, Executive at AbbVie and Guest Author at Cytel...
Read article

November 27, 2019
Interview with David Kerr: Data Monitoring Committees (DMCs) – Behind Closed Doors
At the 2019 Challenges in Rare Diseases Clinical Trials Symposium and East training, Cytel partnered with Alexion to...
Read article

November 21, 2019
Biotechs and Medtechs, don’t forget your market access strategy (part 2 of 4): The critical role of market access planning in clinical development
Author: Michael S. Paas, Market Access & Commercialization Expert, Executive at AbbVie and Guest Author at Cytel In my...
Read article

November 14, 2019
Biotechs and Medtechs, don’t forget your market access strategy (part 1of 4): Why is market access strategy crucial to succeed?
Market access strategy is an integral part of the clinical development process to ensure success in global healthcare...
Read article

November 5, 2019
Drug Development in Rare Diseases - Innovation in Statistical Thinking
Cytel is delighted to have Kannan Natarajan speaking at the “Complex Innovative Trial Design Symposium and East User...
Read article
October 10, 2019
The Challenges of Rare Diseases in Clinical Trials Symposium and Hands-on East Training
A disease is generally considered to be rare if it affects one patient per 200,000 people (1) and most rare diseases...
Read article

August 23, 2019
Advancing Medicines Development with External Controls
In place of collecting data from patients recruited for a trial who have been assigned to the control or...
Read article

August 14, 2019
Keeping Clinical Trials on Track: A Statistician's Perspective
This article was originally published as part of a series by pharmaphorum in association with Cytel and is reproduced...
Read article

August 7, 2019
Career Perspectives: Jayshree Garade
In this blog, from our career perspectives series, we talk with Jayshree Garade Associate Director, Statistical...
Read article

August 1, 2019
Predictive Biomarker Signature Characterization
The term biomarker signature describes the behavior of a set of biomarkers that define a signature to maximize the...
Read article

July 22, 2019
Adaptive Design and Health Economic Analysis: Interview with Laura Flight
Health economics and adaptive design methods share common ground in that they both aim to support more efficient and...
Read article

July 8, 2019
Estimands are not just a statistical issue- Q&As and webinar replay
Cytel recently hosted a very well-attended and engaging webinar on the topic of “Estimands, not just a statistical...
Read article

June 27, 2019
Handling the specialized data requirements in oncology clinical trials
By Nicolas Rouillé and Eric Henniger The right design and the right data ultimately leads to the right decisions, so...
Read article

June 14, 2019
Flipping the paradigm-how should biotechs harness adaptive trials?
This article was originally published as part of a series by pharmaphorum in association with Cytel and is reproduced...
Read article



March 26, 2019
How Patient-Reported Outcomes Improve Outcomes
At the Partnerships in Clinical Trials Conference in Barcelona in November 2018, Strategic Consultant Ursula Garczarek...
Read article

March 20, 2019
Operation Rescue: Addressing Lagging Trials
No one plans to have a trial whose data collection needs rescuing. However, lagging enrollment rates, operational...
Read article

March 15, 2019
Strategic Applications of Pharmacometrics in Clinical Development
Quantitative pharmacology encompasses the many strategic advantages of using complex mathematical models to understand...
Read article

February 28, 2019
Statistical Approaches to Overcome Challenges in Rare Disease Development
In honor of Rare Disease Day 2019 we share a new Cytel podcast featuring Cytel Strategic Consultant Ursula Garczarek...
Read article

February 21, 2019
Podcast with Ursula Garczarek on The Effective Statistician
In 2018, Cytel ran a qualitative survey among biostatisticians and programmers on trends in data science and...
Read article

February 8, 2019
Publication Reveals New Promise for Promising Zone Designs
A 2018 publication in the Biometrical Journal by Cytel’s Cyrus Mehta, Lingyun Liu and Sam Hsiao, ‘Optimal Promising...
Read article

January 30, 2019
Career Perspectives: Interview with Tina Checchio, Associate Director, Quantitative Pharmacology & Pharmacometrics
QPP remains at the heart of model based drug development. Short for Quantitative Pharmacology & Pharmacometrics, it...
Read article
January 10, 2019
Podcast: Overcoming Phase 1 Development Challenges
Nand Kishore Rawat is a Director and Head, Early Phase Biostatistics based in the King of Prussia, PA Cytel office. We...
Read article

November 12, 2018
Can Statisticians Contribute to Enhance the Position of Patients in Clinical Trials?
In this blog, we talk with Robert Greene, Founder and President of the HungerNDThirst Foundation, about his upcoming...
Read article

November 8, 2018
Data Management Fundamentals for Your Next Clinical Trial
Data is the most crucial asset in any clinical trial and is used to ultimately drive the decision-making process...
Read article

November 5, 2018
New publication addresses critical issues in ultra-orphan indications
Cytel biostatisticians Cyrus Mehta and Lingyun Liu, together with Charles Theuer, CEO of TRACON Pharmaceuticals have...
Read article


October 23, 2018
Career Perspectives: Interview with Munshi Imran Hossain, Senior Data Scientist
Cytel data scientists apply advanced statistical techniques including predictive modeling of biological processes and...
Read article


October 5, 2018
Interview with Stephen Senn: 70 Years and Still Here: The Randomized Clinical Trial and its Critics
We are delighted that Stephen Senn will be joining us at the EUGM on November 14th and 15th in Darmstadt, Germany. In...
Read article

September 27, 2018
Decision Making in Development Programs with Targeted Therapies: with Heiko Götte
In this blog, we talk with Heiko Götte, Senior Expert Biostatistician at Merck about his upcoming presentation at...
Read article

September 20, 2018
Career Perspectives: Interview with Adam Hamm, Director of Biostatistics
At Cytel we believe that expert statistical input has the power to shape the future of clinical development: de-risking...
Read article

September 10, 2018
Could data science be about to revolutionize the regulatory approval of new drugs?
The biopharmaceutical and healthcare industries now collect more data than ever before due to advances in the variety...
Read article

September 7, 2018
Opportunities of FDA’s Innovative Trial Design Pilot Meeting Program
On August 29th 2018, the FDA announced (1) that it would be establishing a Complex Innovative Trial Design (CID) Pilot...
Read article

September 5, 2018
Podcast: Enhancing Patient Enrollment Forecasting with EnForeSys 2.0
EnForeSys is Cytel’s tool for patient recruitment planning. We have discussed on the blog recently with Tufts...
Read article

August 31, 2018
Highlights from the JSM 2018 Conference
JSM 2018, ASA’s annual gathering of over 6500 attendees attracted statisticians and data scientists to the beautiful...
Read article

August 23, 2018
Career Perspectives: Interview with Meredith Alm, Manager, QA Compliance
Cytel has grown significantly over the last 30 years, with operations across North America, Europe, and India. All of...
Read article

August 15, 2018
2018 East User Group Meeting Addresses Multiplicity Themes, with keynotes including Stephen Senn and Meinhard Keiser.
Cytel’s 7th East User Group Meeting (EUGM) will take place on November 14 & 15, 2018 at Merck in Darmstadt, Germany,...
Read article
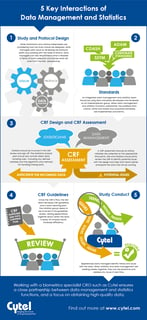
July 27, 2018
Infographic: 5 Key Interactions of Data Management and Statistics
In this blog, we share a new infographic based on this popular blog post illustrating some of the critical interactions...
Read article

July 24, 2018
Career Perspectives: Interview with Sam Hsiao, Associate Director, Strategic Consulting
At Cytel our strategic consulting team works on a wide range of projects including: Identifying the best clinical trial...
Read article

July 18, 2018
Recent Publication: On shapes of ADR report accumulation data
A recent article published by Cytel authors Samadhan Ghubade, Sharayu Paranjpe, Kushagra Gupta, Anil Gore and colleague...
Read article

July 16, 2018
Case Study:Creating an Effective Functional Services Partnership
In this blog we share a case study of how we established and ramped up a functional service outsourcing partnership for...
Read article

July 3, 2018
Unveiling New East 6.5 Modules: Join Our Webinar
It’s shaping up to be a busy year for Cytel’s software development team with a number of upgrades and planned launches...
Read article

July 2, 2018
Highlights from the PSI 2018 Conference
A number of the Cytel team were in Amsterdam, 3rd- 6th June 2018 for the PSI Conference. This year’s conference was...
Read article

June 28, 2018
Non-Compartmental Analysis and the Early Phase Regulatory Environment
By Esha Senchaudhuri With thanks also to Jitendarreddy Seelam and Ramanatha Saralaya for their input. The fact of the...
Read article

June 19, 2018
The Importance of Standardization in Clinical Outsourcing
At the recent PCMG conference in Malta, Adrian Otte ( Independent Consultant, formerly VP Global Development Operations...
Read article

June 14, 2018
What makes a good data manager?
In this blog, Paul Fardy, Executive Director of Data Management at Cytel shares his thoughts on how the data manager...
Read article

May 31, 2018
5 Reasons to Integrate MBMA Into Your Clinical Development Strategy
By Esha Senchaudhuri An important trend in clinical development involves integrating strategic pharmacometric analysis...
Read article

May 23, 2018
Addressing Critical Unmet Oncology Needs in the Era of Precision Medicine
Our Industry Voices series showcases our clients’ innovative work and breakthrough therapeutics in oncologic...
Read article

May 16, 2018
Rewriting the oncology textbook with cell-based immunotherapies
Our Industry Voices series showcases our clients’ innovative work and breakthrough therapeutics in oncologic...
Read article

May 9, 2018
Innovative Oncology Trial Designs in Practice
As we prepare to head to ASCO in under a month's time, we are pleased to share a new ebook that showcases some key...
Read article

April 25, 2018
Overcoming Data Management Challenges in Immuno-Oncology Trials
Data management is an essential building block for successful Immuno-Oncology (I-O) trials. At the Immuno-Oncology...
Read article
April 18, 2018
Developing the Next Generation of Skills for Statistical Programmers
Our recent Clinical Biometrics Survey explored the views of respondents from across the statistical programming,...
Read article

April 13, 2018
Exploring Challenges of Clinical Trial Operations Part 2 with Ken Getz
We return to our discussion with Ken Getz of the Tufts CSDD for part 2 of our blog post on key challenges in clinical...
Read article

April 5, 2018
Ken Getz: Exploring Challenges of Clinical Trial Operations
Photo by J. Kelly Brito on Unsplash Research on clinical trial enrollment makes for sobering reading, characterized by...
Read article

April 3, 2018
Maximizing Preclinical Knowledge for Optimal R&D
By Esha Senchaudhuri In response to its R&D productivity from 2005 – 2010, AstraZeneca took the initiative in 2011 to...
Read article

March 16, 2018
Career Perspectives: Interview with Benjamin Esterni, Principal Biostatistician
At Cytel we believe that expert statistical input has the power to shape the future of clinical development: de-risking...
Read article

February 28, 2018
Insight into the Coordination of Rare Diseases at Sanford registry
There is a consensus in the industry that data on rare diseases is limited, incomplete, and difficult to find or...
Read article

February 27, 2018
Congrats to Lipopharma and CLINGLIO Consortium on Recent Grant Award
We extend our congratulations to Lipopharma and the CLINGLIO project consortium on their recent 6,15M€ grant award by...
Read article

February 15, 2018
A Gatekeeping Procedure to Test a Group Sequential Design
A recent publication in Biometrics ‘A Gatekeeping Procedure to Test a Primary and a Secondary Endpoint in a Group...
Read article

February 13, 2018
Career Perspectives: Interview with Ursula Garczarek, Associate Director - Strategic Consulting
Our strategic consulting team work on projects such as: Identifying the best clinical trial design, implementing...
Read article

February 6, 2018
Life in Programming: Interview With Ajay Sathe
We were excited to learn recently that Ajay Sathe, the CEO of our India Operations, was awarded lifetime honorary...
Read article

January 26, 2018
6 Innovative Trial Design Videos
The Cytel YouTube Channel hosts a wealth of video presentations from Cytel experts as well as external industry and...
Read article

January 23, 2018
Interview: Promoting precision medicine using data science
News Medical interviewed Dr. Rajat Mukherjee, Statistician, and Director of Data Science at Cytel to investigate the...
Read article

January 9, 2018
Career Perspectives: Interview with Lisa Goldberg, Associate Director of Statistical Programming
Our Career Perspectives' series is back! Cytel has industry-leading experts in statistical programming with years of...
Read article


November 28, 2017
Interview: Clinical Trial Optimization with R
In this blog we turn to some reading matter, and interview Gautier Paux and Alex Dmitrienko about the recent book...
Read article

November 22, 2017
Career Perspectives: Interview with Makarand Deshmukh, Senior Clinical Data Analyst
Cytel offers a full range of clinical data management services and the team of experts is spread across the globe. In...
Read article

November 15, 2017
Creating Efficiencies in the Vendor Qualification Process: A Proposal
Each year Halloran Consulting Group hosts‘CORE’ (Clinical Operations Retreat for Executives) as a forum for industry...
Read article

November 9, 2017
The Cytel Story: In the Co-Founders' Own Words
In this blog we are excited to unveil a new project which we have been hard at work on over the last few months. 2017...
Read article
November 6, 2017
Asking the Right Questions of Your Data: Experiences in Model Informed Drug Development
At the Chief Medical Officer Summit earlier this year, Cytel's Director of Quantitative Pharmacology and...
Read article

October 31, 2017
Webinar Replay: Dual Target Methods for Go/No-Go Decision Making
As part of Cytel's new Trial Innovations Webinar Series, Pat Mitchell, Statistical Science Director at AstraZeneca...
Read article

October 27, 2017
Design Concept for Confirmatory Basket Trial Interview with Bob Beckman: Part 2
In this blog, we share the second part of our interview with Bob Beckman, about a design concept for a confirmatory...
Read article

October 18, 2017
Webinar Replay: Phase 2 Trial Designs using Program-level Simulations
Cytel's new Trial Innovations Webinar Series provides a platform for the most promising new statistical approaches...
Read article

September 29, 2017
Career Perspectives: Interview with Namrata Deshpande, Senior Team Lead
Namrata Deshpande, Senior Team Lead will be participating in a round table discussion at the Women in Statistics event...
Read article
September 20, 2017
Accurate Event Prediction in a Cardiovascular Outcomes Research Trial
In this blog we share a case study of work our strategic consulting team conducted supporting accurate event prediction...
Read article

September 13, 2017
How can Novel Statistical Methods Tackle Antibiotic Resistance?
Antibiotic resistance is one of the greatest challenges facing human health today. We are excited to welcome Dr. Scott...
Read article

September 11, 2017
Design Concept for Basket Trials: Interview with Bob Beckman
At the East User Group meeting (EUGM) on 25th and 26th October, we will welcome a number of renowned industry speakers...
Read article

August 10, 2017
4 Questions to Explore in Model-Informed Drug Development (Infographic)
Model-informed drug development has been defined by Richard Lalonde ( Lalonde, 2007) (1) as “Development and...
Read article

August 2, 2017
Case Study: Cross-study Efficiencies in Biometrics Outsourcing
As a biometrics -focused CRO, Cytel regularly works across a program of studies, providing data consistency, and...
Read article

July 26, 2017
Are Adaptive Designs the Answer to Oncology Development Success?
Sadly, clinical development of anti-cancer therapeutics faces particularly high rates of failure, even in the context...
Read article
July 19, 2017
Case Study: Seamless Independent Data Monitoring Committee Support
With adaptive and innovative trial designs on the rise, operational implementation of interim analyses, including...
Read article

July 13, 2017
Creating Data Visualizations with R and Shiny
By Tejasweeni Rajput It’s been known for centuries that a picture can tell a thousand words. In an era of new...
Read article

July 11, 2017
Collaboration Brings Success for the UK Adaptive Designs Working Group.
The Adaptive Designs and Multiple Testing Procedures Workshop (ADMTP), the first joint meeting of the Adaptive Designs...
Read article
July 6, 2017
When Biostatisticians Disagree About Ethics
By Esha Senchaudhuri The ethical benefits of adaptive clinical trials have been widely acclaimed: higher prospects for...
Read article
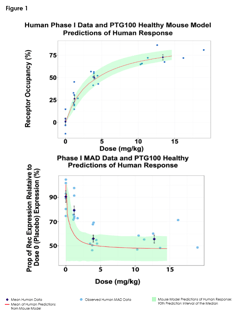
June 12, 2017
Predictions of Pharmacodynamic Responses in Ulcerative Colitis Patient
The Population Approach Group in Europe (PAGE) represents a community with a shared interest in data analysis using the...
Read article
June 8, 2017
Measuring Intergroup Agreement and Disagreement
Cytel's Madhusmita Panda presented at this year’s PSI Conference in the Innovative Methodology session on the topic of...
Read article

May 30, 2017
Interview: How can a Bayesian framework support benefit risk assessment?
A recent paper The case for Bayesian methods in benefit-risk assessment: Overview and future directions (1) co-authored...
Read article

May 26, 2017
Cytel statistical programmer gains recognition at PharmaSUG 2017
PharmaSUG 2017 proved to be an inspirational and informative event. With over 200 paper presentations, posters, and...
Read article

May 22, 2017
Jim Bolognese named 2017 American Statistical Association Fellow
James (Jim) Bolognese, Senior Director, Strategic Consulting, Clinical Services at Cytel Inc. was named a 2017 fellow...
Read article

May 17, 2017
Case Study: From Trial Design to CDISC Submission
This new case study shares how Cytel supported a specialist biopharmaceutical company from Phase 2 trial design through...
Read article

May 4, 2017
The Data Management Perspective on the Interim Analysis
As a recognized expert in adaptive trials, Cytel has extensive experience designing and managing trials with interim...
Read article
April 28, 2017
Case Study: Redesigning a Pragmatic Trial in Oncology
In this blog we share a case study in which our statistical consulting team helped a client redesign an oncology...
Read article

April 25, 2017
Critical Operational Considerations for Interim Analyses
At a recent conference Adam Hamm, Director Biostatistics at Cytel, presented his thoughts on Best Practices and...
Read article

April 11, 2017
FDA 22 Case Studies and Mitigating Phase 3 Risks
In a January 2017 paper (1), the FDA reviewed 22 case studies where promising Phase 2 trials did not result in...
Read article

April 5, 2017
Better Management and Outputs in Statistical Programming
Statistical programmers at all levels can make a significant impact on streamlining delivery, improving efficiency, and...
Read article

March 29, 2017
New Publication: Design and Monitoring of Multi-Arm Multi-Stage Clinical Trials
With an increasing interest in platform designs and other innovative designs that involve multiple comparisons over...
Read article
March 24, 2017
Case Study: Improving Go/No-go Decision-Making with Custom Software
Robust go/no-go (GNG) decision-making is essential for effectively managing risk across a clinical portfolio. In early...
Read article

March 15, 2017
The Data Management Plan Takes Center Stage- why is it so important?
A precise and thorough approach to planning is key for success in data management. The Data Management Plan (DMP) is a...
Read article

March 10, 2017
Flexible approaches to Biosimilars Development
At the recent Biosimilars Summit in Philadelphia, Cytel's Pantelis Vlachos presented on statistical challenges and...
Read article

March 2, 2017
Case Study: Bayesian Decision-Making in a Phase 3 Oncology Design
We continue our case study series with this example of a Phase 3 design that uses Bayesian decision making combined...
Read article

February 27, 2017
Estimands 101: Interview with Mouna Akacha
It’s been hard to miss the prevalence of estimand-related discussions in the last year. This is a topic which is very...
Read article

February 21, 2017
Syntax and Variables in R: A Primer
In a previous blog, we provided an overview of basic data structures in R. In this follow up piece, we will provide a...
Read article

February 15, 2017
Outsourcing success for emerging biopharma
Outsourcing solutions should never be a one size fits all process, and smaller and emerging biopharma companies may...
Read article

February 6, 2017
The Making of a CDISC Trainer
CDISC is a global, nonprofit charitable organization whose mission is ‘to inform patient care and safety through higher...
Read article

January 30, 2017
Accelerating development with combined SAD/MAD approach
Single ascending dose (SAD) and multiple ascending dose (MAD) studies are typically the first in human studies. They...
Read article

January 23, 2017
How to get the regulatory green light for your adaptive design?
As a group, Cytel had over 40 successful regulatory interactions last year, many of which supported approvals for...
Read article

January 19, 2017
Data Structures in R: A Primer
R is on the rise in biopharma, and as we have previously discussed on the blog, it is now time for SAS programmers to...
Read article

January 16, 2017
Adaptive Design Approaches from Cardiovascular Clinical Trialists Forum
The Global Cardiovascular Clinical Trialists Forum is a key event bringing together leading experts from across the...
Read article

January 5, 2017
SAS and NONMEM - a marriage made in heaven?
Nonlinear Mixed Effects Modeling (NONMEM) is a type of population pharmacokinetics/pharmacodynamics (popPK/PD) analysis...
Read article

December 21, 2016
CDISC submissions- are you up to speed?
December 18th 2016 was a significant date for the pharmaceutical industry and regulatory submissions. For trials which...
Read article

December 20, 2016
How do CDASH standards build data quality?
Data Standards play a crucial role in structuring and promoting long term value of clinical data. Clinical Data...
Read article

December 15, 2016
Adaptive Design CONSORT Extension Project: The Inside Scoop
In April, we interviewed NIHR research fellow Munya Dimairo about the paper, ‘Adaptive designs undertaken in clinical...
Read article

December 6, 2016
Ensuring quality data no matter the phase: data management considerations
The management of quality clinical data collection is built on a number of core essentials- including project...
Read article
December 2, 2016
Innovative Phase 3 Adaptive Enrichment Design in Oncology
At a recent Pfizer/ Cytel seminar on rare disease and oncology development, Cytel’s Lingyun Liu presented innovative...
Read article

November 21, 2016
Infographic: 10 steps to consider before choosing an adaptive design
While adaptive designs can deliver significant benefits to clinical development- including ethical benefits for...
Read article

November 18, 2016
Case Study: Dose-response modeling informs Phase 2 ulcerative colitis study design
Challenge Our client had the following key questions which they wanted our pharmacometrics group to address for an...
Read article
November 14, 2016
Infographic: 9 Do's and Don'ts to Ensure Independence of QC
In our last blog, we shared some of Angelo Tinazzi and Cedric Marchand's recommendations on how to ensure independence...
Read article

November 11, 2016
How to ensure independence of QC in statistical programming
A solid and robust QC process is one vital component of ensuring quality programming delivery. Angelo Tinazzi and...
Read article

November 9, 2016
New East Insights Video:Creating an SSR Design
Adaptive sample size re-estimation designs are an important part of the statistician's toolkit. In this first in a...
Read article
November 1, 2016
Pharmacometrics tools of the trade: 4 factors to consider
Unlike statistics which has been around in some form for hundreds of years, pharmacometrics is, by comparison, a...
Read article
October 25, 2016
R Beyond Statistics
Use of R is a hot topic among statisticians and programmers in the pharmaceutical industry. At the recent PhUSE...
Read article
October 13, 2016
The evolving role of the modern statistical programmer
Statistical programmers play a key role in turning the data from clinical trials into knowledge and supporting the...
Read article

October 11, 2016
Simulations to optimize clinical trial programs
Its important to take a strategic approach to clinical development in order to minimize the potential for Phase 3...
Read article

October 7, 2016
Adaptive Designs: A Data Management Perspective
Adaptive designs have the potential to accelerate clinical development, and improve the probability of trial success....
Read article
October 5, 2016
Challenges in Neuroscience Clinical Trials
While some progress has been made in terms of scientific development in Neuroscience and Neuropsychiatry indications,...
Read article

September 29, 2016
Case studies:Learning from less-well understood adaptive designs
A paper "Best practices case studies for 'less well-understood' Adaptive designs", has been published by the DIA...
Read article

September 27, 2016
Practical Challenges of the LUNG-MAP study
The Lung-MAP trial is an innovative biomarker driven 'precision medicine' study which evaluates five novel agents for...
Read article
September 20, 2016
An efficient tool for model based meta-analysis
Drug development is an expensive and risky business. To maximize a compound’s ultimate chances of commercial as well as...
Read article

September 13, 2016
Case Study:Exposure Response Modeling in Hematology
Exposure-response data gained from clinical studies can provide a basis for model-based analysis and simulation,...
Read article
September 9, 2016
Case Study:Seamless Phase 2/3 Design in Rare Disease
Challenge: Our client, an emerging biotechnology company, was preparing for the next stage of development for their...
Read article

September 7, 2016
Overcoming challenges of 'Less Well Understood' Adaptive Designs
In the 2010 draft FDA ‘Guidance for Industry on Adaptive Design Clinical Trials for Drugs and Biologics', the agency...
Read article
August 31, 2016
An Introduction to BLRM
Traditional rule-based approaches to dose escalation such as 3+3 are widely used in early clinical development. They...
Read article

August 26, 2016
Sample Size Re-Estimation in Bioequivalence Trials with Small Samples
At the recent JSM in Chicago, Cytel’s Sam Hsaio and Lingyun Liu alongside Genentech's Romeo Maciuca, presented a...
Read article

August 23, 2016
How does the T-Statistic stack up for finding MTD?
At the recent JSM meeting in Chicago, Cytel's Jim Bolognese presented the results of work he has conducted evaluating...
Read article

August 17, 2016
Adaptive Designs: In Conversation with the NEJM
Following the recent publication of their review article Adaptive Designs for Clinical Trials in the New England...
Read article
August 9, 2016
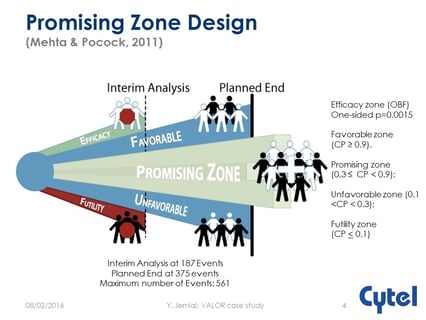
Operational and regulatory considerations in a promising zone trial
At the recent JSM Meeting, Cytel’s Yannis Jemiai presented the case study of the VALOR trial which used a promising...
Read article
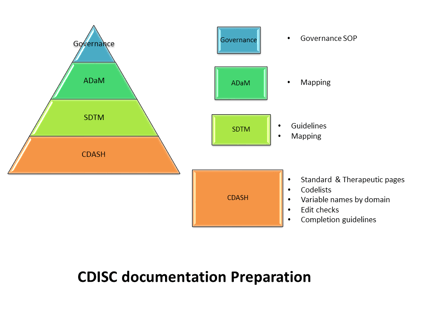
August 2, 2016
The CRO role in Data Standards Governance
Editor's note( this blog was refreshed in April 2018) As CDISC compliant submissions become increasingly expected,...
Read article
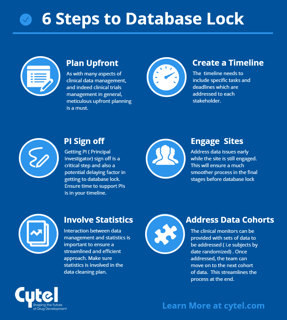
July 28, 2016
6 steps to timely database lock
To close a clinical database right the first time you have to begin with study start-up. Clearly, you can’t close a...
Read article

July 25, 2016
5 trends a statistical programmer needs to follow
Statistical programmers are in high demand within the biopharmaceutical industry, and within the dynamic world of...
Read article

July 21, 2016
Webinar Replay: Single and Double Agent Dose Escalation Designs
Did you miss our webinar on Single and Dual Agent Dose escalation designs earlier in the year? In this blog we have...
Read article

July 18, 2016
Adaptive Design in the limelight with NEJM article
In order for adaptive designs to reach their potential, it’s critical that knowledge is effectively dissemirnated...
Read article

July 12, 2016
Key considerations in selecting an EDC system
How do you go about selecting the best Electronic Data Capture (EDC) system for your study? There is now a vast amount...
Read article

July 7, 2016
Wild Horses: How StatXact is helping conservation project in Mongolia
Why do we do what we do? At Cytel we have always been driven to deliver benefits in the service of human health, and...
Read article
July 5, 2016
PROC MCPMod in Bronchodilator Case Study
At a recent PhUSE SDE, Cytel’s Chitra Tirodkar presented how East PROC MCPMod could be used to help solve the problem...
Read article


June 23, 2016
Getting the best out of your biometrics RFP
Vendor selection is a critical component of ensuring clinical trial success. A 2015 report (1) suggested that clinical...
Read article
June 21, 2016
Sharpening your Advanced SAS Skills: Interview with Sunil Gupta
Sunil is an Associate Director of Statistical Programming at Cytel and has over twenty-five years of experience in the...
Read article

June 16, 2016
Determining the future course of your trial
Predicting the course of a clinical trial is something which people will always want to do-whether for statistical...
Read article
June 14, 2016
Managing DMC analysis- an innovative programming solution
At Cytel, we are very often asked to get involved in DMCs ( Data Monitoring Committees) in a variety of capacities. Our...
Read article

June 7, 2016
How can the CMO/Biostatistician connection improve clinical development?
At the recent CMO Summit East James ( Jim) Bolognese, Cytel’s Senior Director of Strategic Consulting, and Lou...
Read article

May 31, 2016
EAST 6.4 Release: Interview with Yannis Jemiai
Last week, we were delighted to announce the release of East 6.4 bringing further cutting –edge approaches to the East...
Read article
May 19, 2016
Pattern Recognition and 'Big Data'
The explosion in healthcare information and “big data “has been one of the most written about topics in the last few...
Read article

May 17, 2016
Scrambled Data – A Population PK/PD Programming Solution
Cytel participated at PharmaSUG 2016 in Denver recently. A key event on the statistical programming global calendar,...
Read article

May 12, 2016
Lost in Traceability- From SDTM to ADaM
Once upon a time Hansel and Gretel laid a trail of breadcrumbs which they followed to find their way back home. Their...
Read article

May 10, 2016
Subgroup Analyses in Early Phase Clinical Trials
We were fortunate to welcome Björn Bornkamp of Novartis to the EUGM 2016 presenting work he has developed jointly with...
Read article

April 28, 2016
Adaptive Designs in Practice
Adaptive Designs in Practice: Interview with NIHR Research Fellow Munya Dimairo NIHR and University of Sheffield...
Read article
April 26, 2016
Overcoming Data Management Challenges in Oncology Studies
In this blog we’ll highlight some unique challenges that are encountered from a Data Management perspective when...
Read article

April 22, 2016
Dual Agent Dose Escalation Designs
FDA draft guidance on “Co development of two or more unmarketed investigational drugs for use in combination” notes...
Read article

April 20, 2016
Handling CDM data integrations
During the course of any clinical trial, there are often data which, while collected electronically, are outside of the...
Read article

April 15, 2016
5 Key Interactions of Clinical DM and Statistics
It's critical for biostatistics and data management to be closely aligned and working effectively together. The...
Read article

April 13, 2016
HTAs: Adjusting Overall Survival for Treatment Switch
We continue our series of blogs covering the expert presentations from the EAST User Group Meeting. Consultant Claire...
Read article
April 7, 2016
Blinded SSR in early phase biosimilar studies
Francois Beckers, Global Head of Biostatistics & Epidemiology at Merck KGaA joined us at the East User Group Meeting in...
Read article
April 5, 2016
What's the price of pharma innovation?
Cost of pharmaceutical development and R&D productivity is an ongoing industry concern, consistently discussed in the...
Read article

March 29, 2016
Decision Making in Early Clinical Development
On March 16th and 17th the 5th East User Group Meeting took place in London. This very successful 2 days saw a variety...
Read article
March 23, 2016
What's the Value of P?
“How many statisticians does it take to ensure at least a 50% chance of a disagreement about p-values?” So questions...
Read article

March 11, 2016
EAST takes on Multi-Arm Multi-Stage Designs
There has been increasing interest in multi-arm multi-stage trials with treatment selection and sample size...
Read article

March 8, 2016
Mind the Gap! How to prepare for SDTM migrations.
Data standardization is critical to ensure successful regulatory submissions. While many sponsors now choose to create...
Read article
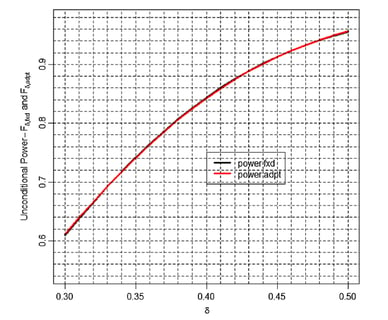
March 3, 2016
Adaptive SSR: Debunking the inefficiency myth
'The aim of a discussion should not be victory but progress.' This principle, expressed by the French essayist Joseph...
Read article

February 26, 2016
Getting Technical: The evolving role of the Data Manager
Remember the early days of Electronic Data Capture? Those first systems, which were revolutionary for their time...
Read article
December 1, 2015
Making the Most of Data Management for Risk Based Monitoring
Risk based monitoring is a strategic monitoring practice which aims to shine the spotlight on problematic study areas,...
Read article
November 12, 2015
2 Talks on Early Phase Go/No-GO Decision Making
Last week Cytel joined forces with Sanofi/Genzyme to devote a full day of workshops and talks related to modern methods...
Read article
November 10, 2015
Bayesian Dose Escalation Designs for Late Onset Toxicity
Last week Cytel joined forces with Sanofi/Genzyme to devote a full day of workshops and talks related to modern methods...
Read article
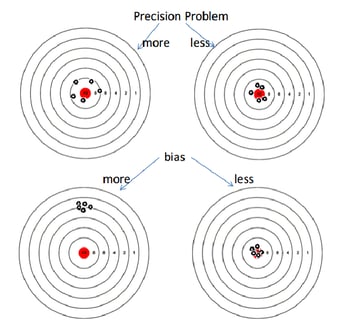
October 29, 2015
How to Reduce Bias in the Estimates of Count Data Regression
A common challenge of working with small sample sizes is determining proper bias correction methods when evaluating a...
Read article

October 15, 2015
MCPMod: An Introduction
The method for dose-response modeling that is widely called MCPMod allows a sponsor to measure the likelihood that...
Read article
October 13, 2015
Statistical Primer for Cardiovascular Research
In honor of World Obesity Day (celebrated on Oct. 11 2015) here is an American Heart Association Statistical Primer on...
Read article
October 8, 2015
Being a Statistician: An Art, a Science or Just Job?
A recent American Statistical Association conference featured a town hall meeting to discuss the role of the...
Read article
October 5, 2015
2 Methods for Evaluating Biomarker Subpopulations | Cytel
One consideration every sponsor of a biomarker-stratified confirmatory trial must take into account, is whether to...
Read article

September 29, 2015
5 Skills Needed by All Highly Effective Statisticians
The Head of the DIA’s Adaptive Design Working Group Asks Us to Consider 5 ‘Soft-Skills’ All Effective Statisticians...
Read article


September 4, 2015
Using Simulation for Accelerated Early Phase Drug Development
Our Client's Challenge: Can knowledge of the relationship between biomarkers and clinical endpoints help us to optimize...
Read article
September 1, 2015
Modern Early Phase Clinical Trial Design Primer
If you’re in the practice of conducting early phase clinical trials, you’ve probably heard that modern trial designs...
Read article

June 25, 2015
3 Strategies to Combat Inferential Conservatism in Small Sample Sizes
When conducting a clinical trial with small or sparse data sets, statistical methods meant for large sample sizes may...
Read article

April 13, 2015
New Cytel Whitepaper: Monte Carlo Simulations for Patient Recruitment
Cytel has published a new whitepaper on Monte Carlo Simulations for Patient Recruitment, which illustrates how a...
Read article
February 12, 2015
Data-Driven Trial Planning: An Interview with Pfizer's Chris Conklin
Data driven decision-making can ensure that every feasibility team achieves its enrollment milestones. By transforming...
Read article
February 3, 2015
Clinical Development & Statistical Methodology for Cardiovascular Risk Assessment
A new publication co-authored by Cytel Co-Founder and President Cyrus Mehta considers a range of clinical development...
Read article
December 2, 2014
How to Incorporate New Technology into Your Clinical Development Strategy
During a recent DIA webinar on reinventing the clinical trial, Laurie Halloran (President of the Halloran Consulting...
Read article
October 30, 2014
Michael Proschan on Blinded Adaptations, Permutations and t-tests
During last week’s East Users Group Meeting, Michael Proschan of the NIH and NIAID, gave a presentation on ‘Blinded...
Read article
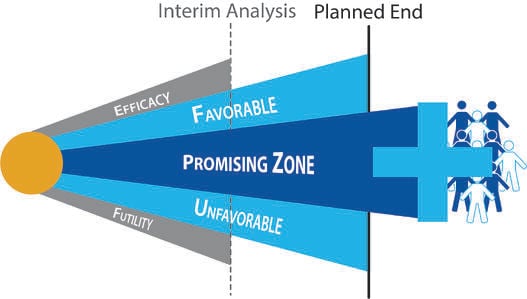
May 13, 2014
De-Risking Drug Development using Adaptive Design
The VALOR trial recently applied a promising zone design to a Phase 3 evaluation of Vosaroxin, a candidate for the...
Read article


