Adaptive Clinical Trial Strategies for the Limited Early Phase Budget
The Journal of the American Medical Association recently published an article entitled ‘The Anatomy of Medical Research: US and International Comparisons.’ The stated objective of the study was to “quantify total public and private investment and personnel (economic inputs) and to evaluate resulting patents, publications, drug and device approvals, and value created (economic outputs)“ [1]
Amongst the many findings of this comprehensive study, a vital observation is the reduction of early phase spending by about 4% per year from 2004 to 2012. One attribution for this decline involves the financial constraints placed upon proof-of-concept trials, particularly when compared to the expected financial benefits of Phase 3 trials and medical devices. According to the authors, “Many new basic discoveries that have probable clinical value are stymied by financial constraints at the critical proof-of-concept stage, where utility in humans is demonstrated.” [1] They add that the number of new discoveries that will be underfunded at the proof-of-concept stage is expected to increase.
A strategic move for those hoping to fund such trials would be to take advantage of the diminished development costs delivered by a properly constructed adaptive clinical trial. In particular, an adaptive dose-finding trial can combine with proof-of-concept to reduce both sample size and study length.
Adaptive Dose-Finding Trials
An adaptive dose-finding trial can refer to both early phase trials and seamless phase 2/3 trials, both of which use adaptive designs to secure the right dose. One such early phase trial design combines proof-of-concept with dose-finding for a seamless Phase 1b/2b adaptive clinical trial. In this trial, instead of conducting proof-of-concept and dose-finding sequentially, a Bayesian Go/No-Go rule is used after the proof-of-concept trial. This rule might stipulate that a trial go directly to Phase 2b based on the results of the proof-of-concept trial. It might alternatively recommend a Phase 1b trial based on these results
By using such a trial, it may be possible to cut out the entire Phase 1b dose-finding trial. The figure above shows one such design for a proof-of-concept for a biomarker, but the design can be extended to other trials as well.
Case Study: Adaptive Phase 2 Dose-Finding Design for Proof-of-Concept & Dose-Exploration via Linear Clinical Utility Function
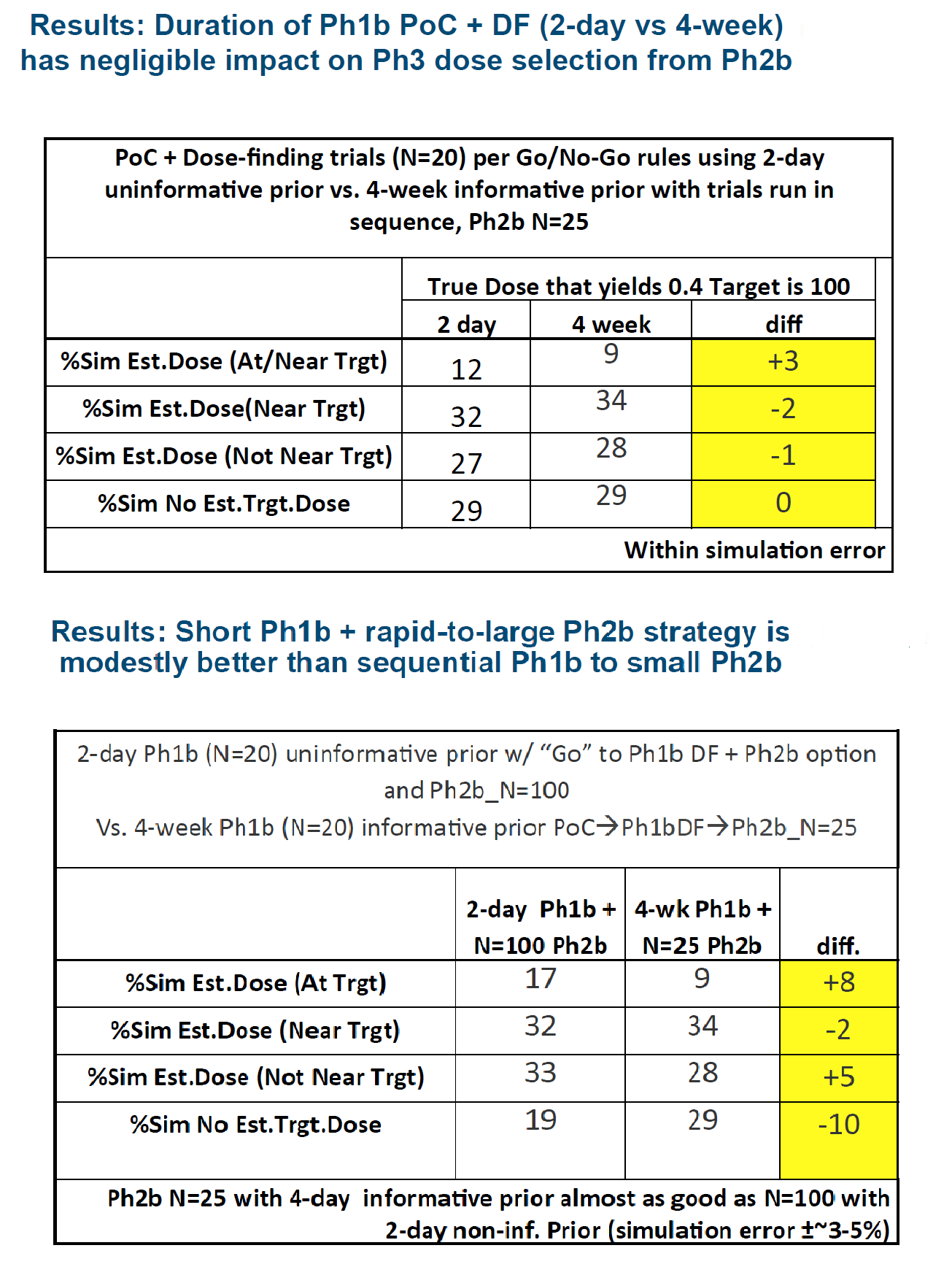
 Cytel Consultant Jim Bolognese recently presented a case study of an Adaptive Phase 2 Dose-Finding Design. The results of his simulations (below) show that by cutting out a Phase 1b trial, trial time can reduce from 4 weeks to 2 days, without loss in accuracy of dose.
Cytel Consultant Jim Bolognese recently presented a case study of an Adaptive Phase 2 Dose-Finding Design. The results of his simulations (below) show that by cutting out a Phase 1b trial, trial time can reduce from 4 weeks to 2 days, without loss in accuracy of dose.
 The findings of this simulation are below. You can also watch a video in which Jim presents this case study. It begins on minute 37.30.
The findings of this simulation are below. You can also watch a video in which Jim presents this case study. It begins on minute 37.30.
Related Items of Interest
[1] Moses, Hamilton, et al. "The anatomy of medical research: US and international comparisons." JAMA 313.2 (2015): 174-189. [Note: You wil need institutional access to read this article]
[2] Early Phase Development Strategy: Bayesian Methods for Go/No-Go Rules
[3] Ranking Adaptive Dose-Finding Designs Using Clinical Utility Functions

